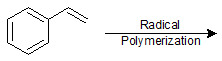Addition of Hydrogen Halides to Alkenes
9-1 Give the IUPAC name for the product of the following reaction.

9-2 Draw the reaction mechanism of the previous problem (9-1).
9-3 Identify the product of the following reactions.
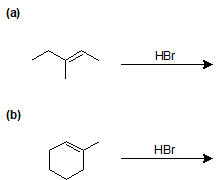
9-4 Identify the products of the following reactions.
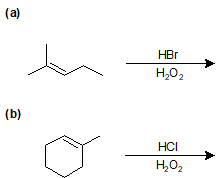
Addition of Water: Hydration of Alkenes
9-5 Identify the product of the following reactions.
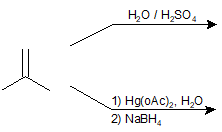
9-6 Identify the product of the following reaction.
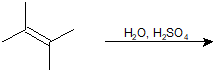
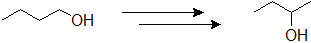
9-7 Propose a plausible route of synthesis for the following product starting with 1-butanol.
9-8 Which of the following alkenes can be used to obtain 3,4-dimethylpentan-2-ol through a hydration reaction using dilute acid?
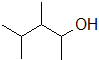
a) (2Z)-3,4-dimethylpent-2-ene
b) 3,4-dimethylpent-1-ene
c) 2,3,4-trimethylpent-2-ene
d) 2,4-dimethyl-3-methylidenepentane
Hydration by Oxymercuration-Demercuration
9-9 Explain why hydration of an alkene by Oxymercuration-Demercuration gives the Markovnikov product.
9-10 Identify the products of the following reactions.

9-11 Identify the product of the following reaction.
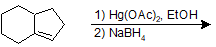
9-12 Propose a possible route of synthesis for the following ether starting with 2-ethylbutan-1-ol.
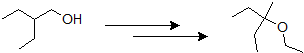
9-13 Give the IUPAC name of the product of the following reaction.
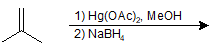
a) 2-methylpropan-2-ol
b) 2,2-dimethylbutane
c) 2-methoxy-2-methylpropane
d) 1,1-dimethylcyclopropane
Hydroboration of Alkenes
9-14 Identify the products of the following reactions.
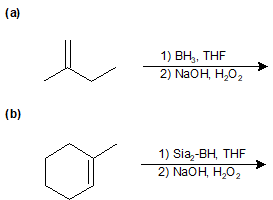
9-15 Identify the products of the following reactions.
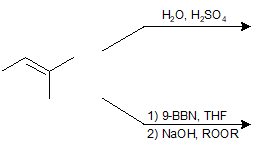
9-16 Give the IUPAC name for the product of the following reaction.
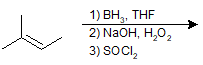
Addition of Halogens to Alkenes
9-17 Identify the products of the following reactions.

9-18 Identify the product of the following reaction.
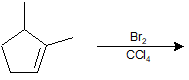
9-19 What is the product of the following reaction?
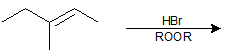
a) (2Z)-2-bromo-3-methylpent-2-ene
b) 2-bromo-3-methylpentane
c) 2,3-dibromo-3-methylpentane
d) (2E)-4-bromo-3-methylpent-2-ene
9-20 What reagents can be used in each step to obtain the following products?
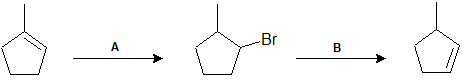
9-21 Explain why you do not obtain a mixture of cis- and trans-brominated products when you react Br2/CCl4 with cyclopentene.
9-22 Identify the product of the following reaction, making sure to include stereochemistry.
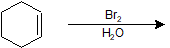
9-23 Draw the mechanism of the reaction in the previous problem (9-22).
9-24 Give the IUPAC name for the product of the following reaction.
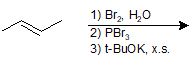
9-25 Identify the product of the following reaction.
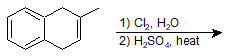
Catalytic Hydrogenation of Alkenes
9-26 Identify the products of the following reactions.
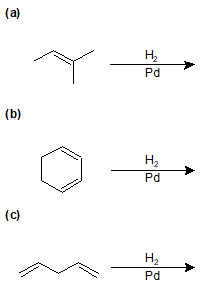
9-27 Suggest a possible route of synthesis, that includes a catalytic hydrogenation step, to obtain the following product.
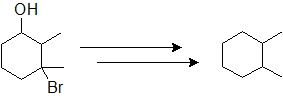
9-28 Identify the product of the following reaction.
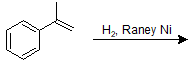
9-29 Identify the product of the following reaction, making sure to include stereochemistry.
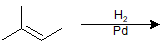
Addition of Carbenes to Alkenes
9-30 Identify the product of the following reaction.
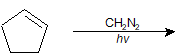
9-31 Identify the product(s) of the following reaction.
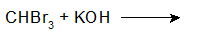
9-32 Identify the product of the reaction when (2E)-3-methylhex-2-ene reacts with the carbene product from the previous problem (9-31), then reacts with Br2 and hv.
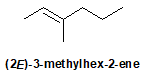
9-33 Propose a possible route of synthesis for the following reaction.

9-34 Identify the product of the following reaction.
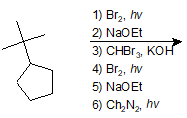
Epoxidation of Alkenes and Acid-Catalyzed Opening of Epoxides
9-35 Identify the product of the following reaction.
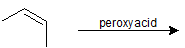
9-36 Identify the product of the following reactions, specifying stereochemistry where appropriate.

9-37 Identify the products of the following reaction, including stereochemistry.
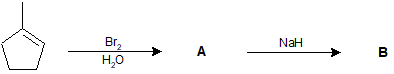
9-38 What is the product of the following reaction?
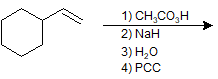
a) benzoic acid
b) 1-cyclohexylethan-1-ol
c) 1-cyclohexylethan-1-one
d) cyclohexanol
9-39 What is the product of the following reaction?
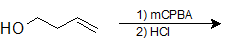
a) 4-chlorobutane-1,3-diol
b) 3-chlorobutan-1-ol
c) 2-chlorobutane-1,4-diol
d) 2,4-dichlorobutan-1-ol
9-40 Draw the arrows for the following epoxidation reaction to show the movement of electrons.
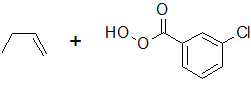
Syn Dihydroxylation of Alkenes
9-41 Identify the product of the following reaction, including stereochemistry.
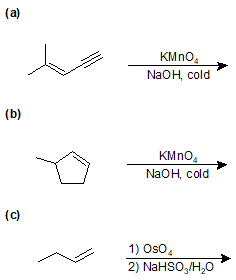
9-42 Give the IUPAC name for the product(s) of the following reaction. Include stereochemistry.
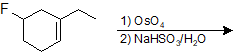
9-43 Identify the product of the following reaction.
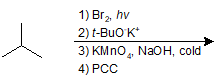
9-44 Suggest a possible route of synthesis for the following compound starting with cyclopentanol.
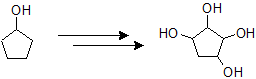
Oxidative Cleavage of Alkenes
9-45 Identify the products of the following reactions.
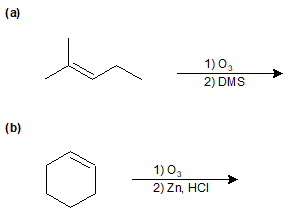
9-46 Identify the products of the following reactions.

9-47 Identify the product of the following reaction.
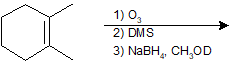
Polymerization of Alkenes
9-48 Identify the alkene monomer that composes the following polymer.
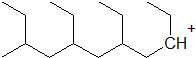
9-49 Draw the mechanism for the acid catalyzed formation of the polymer in the previous problem (9-48).
9-50 Draw the resulting polymer of the following reaction. Draw the chain four monomers in length.