12.6: Buffers
- Page ID
- 17496
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To define buffer and describe how it reacts with an acid or a base.
Weak acids are relatively common, even in the foods we eat. But we occasionally come across a strong acid or base, such as stomach acid, that has a strongly acidic pH of 1–2. By definition, strong acids and bases can produce a relatively large amount of hydrogen or hydroxide ions and, as a consequence, have a marked chemical activity. In addition, very small amounts of strong acids and bases can change the pH of a solution very quickly. If 1 mL of stomach acid [which we will approximate as 0.05 M HCl(aq)] is added to the bloodstream, and if no correcting mechanism is present, the pH of the blood would go from about 7.4 to about 4.9—a pH that is not conducive to continued living. Fortunately, the body has a mechanism for minimizing such dramatic pH changes.
The mechanism involves a buffer, a solution that resists dramatic changes in pH. A buffer (or buffered) solution is one that resists a change in its pH when H+ or OH– ions are added or removed owing to some other reaction taking place in the same solution. Buffers do so by being composed of certain pairs of solutes: either a weak acid plus its conjugate base or a weak base plus its conjugate acid.
For example, a buffer can be composed of dissolved acetic acid (HC2H3O2, a weak acid) and sodium acetate (NaC2H3O2). Sodium acetate is a salt that dissociates into sodium ions and acetate ions in solution. For as long as acetic acid and acetate ions are present in significant amounts a solution, this can resist dramatic pH changes. Another example of a buffer is a solution containing ammonia (NH3, a weak base) and ammonium chloride (NH4Cl). Ammonium acetate is also a salt that dissociates into ammonium ions and chloride ions in solution. The presence of ammonium ions with ammonia molecules satisfies the requisite condition for a buffer solution.
How Buffers Work
The essential component of a buffer system is a conjugate acid-base pair whose concentration is fairly high in relation to the concentrations of added H+ or OH– it is expected to buffer against. Let us use an acetic acid–sodium acetate buffer to demonstrate how buffers work. If a strong base—a source of OH−(aq) ions—is added to the buffer solution, those hydroxide ions will react with the acetic acid in an acid-base reaction:
\[HC_2H_3O_{2(aq)} + OH^−_{(aq)} \rightarrow H_2O_{(ℓ)} + C_2H_3O^−_{2(aq)} \label{Eq1} \]
Rather than changing the pH dramatically by making the solution basic, the added hydroxide ions react to make water, and the pH does not change much.
Many people are aware of the concept of buffers from buffered aspirin, which is aspirin that also has magnesium carbonate, calcium carbonate, magnesium oxide, or some other salt. The salt acts like a base, while aspirin is itself a weak acid.
If a strong acid—a source of H+ ions—is added to the buffer solution, the H+ ions will react with the anion from the salt. Because HC2H3O2 is a weak acid, it is not ionized much. This means that if lots of hydrogen ions and acetate ions (from sodium acetate) are present in the same solution, they will come together to make acetic acid:
\[H^+_{(aq)} + C_2H_3O^−_{2(aq)} \rightarrow HC_2H_3O_{2(aq)} \label{Eq2} \]
Rather than changing the pH dramatically and making the solution acidic, the added hydrogen ions react to make molecules of a weak acid. Figure \(\PageIndex{1}\) illustrates both actions of a buffer.

Figure \(\PageIndex{1}\): The Action of Buffers. Buffers can react with both strong acids (top) and strong bases (bottom) to minimize large changes in pH.
A simple buffer system might be a 0.2 M solution of sodium acetate; the conjugate pair here is acetic acid HAc and its conjugate base, the acetate ion Ac–. The idea is that this conjugate pair "pool" will be available to gobble up any small (≤ 10–3 M) addition of H+ or OH– that may result from other processes going on in the solution.
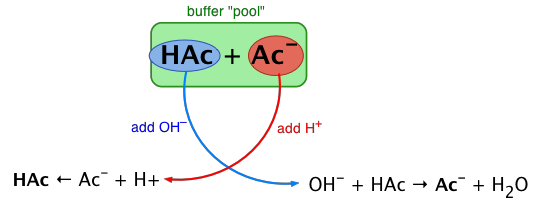
Buffers work well only for limited amounts of added strong acid or base. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes in pH may occur. We say that a buffer has a certain capacity. Buffers that have more solute dissolved in them to start with have larger capacities, as might be expected.
Buffers made from weak bases and salts of weak bases act similarly. For example, in a buffer containing NH3 and NH4Cl, ammonia molecules can react with any excess hydrogen ions introduced by strong acids:
\[NH_{3(aq)} + H^+_{(aq)} \rightarrow NH^+_{4(aq)} \label{Eq3} \]
while the ammonium ion [NH4+(aq)] can react with any hydroxide ions introduced by strong bases:
\[NH^+_{4(aq)} + OH^−_{(aq)} \rightarrow NH_{3(aq)} + H_2O_{(ℓ)} \label{Eq4} \]

Which solute combinations can make a buffer solution? Assume all are aqueous solutions.
- HCHO2 and NaCHO2
- HCl and NaCl
- CH3NH2 and CH3NH3Cl
- NH3 and NaOH
Solution
- Formic acid (HCHO2) is a weak acid, while NaCHO2 is a salt supplying —formate ion (CHO2−), the conjugate base of HCHO2. The combination of these two solutes would make a buffer solution.
- Hydrochloric acid (HCl) is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution.
- Methylamine (CH3NH2) is like ammonia, a weak base. The compound CH3NH3Cl is a salt supplying CH3NH3+, the conjugate acid of CH3NH2. The combination of these two solutes would make a buffer solution.
- Ammonia (NH3) is a weak base, but NaOH is a strong base. The combination of these two solutes would not make a buffer solution.
Which solute combinations can make a buffer solution? Assume all are aqueous solutions.
- NaHCO3 and NaCl
- H3PO4 and NaH2PO4
- NH3 and (NH4)3PO4
- NaOH and NaCl
- Answer
-
b. H3PO4 (weak acid) and H2PO4- (conjugate base of H3PO4) make a buffer.
c. NH3 (weak base) and NH4+ (conjugate acid of NH3) make a buffer
Although medicines are not exactly "food and drink," we do ingest them, so let's take a look at an acid that is probably the most common medicine: acetylsalicylic acid, also known as aspirin. Aspirin is well known as a pain reliever and antipyretic (fever reducer).
The structure of aspirin is shown in the accompanying figure. The acid part is circled; it is the H atom in that part that can be donated as aspirin acts as a Brønsted-Lowry acid. Because it is not given in Table 10.5.1, acetylsalicylic acid is a weak acid. However, it is still an acid, and given that some people consume relatively large amounts of aspirin daily, its acidic nature can cause problems in the stomach lining, despite the stomach's defenses against its own stomach acid.
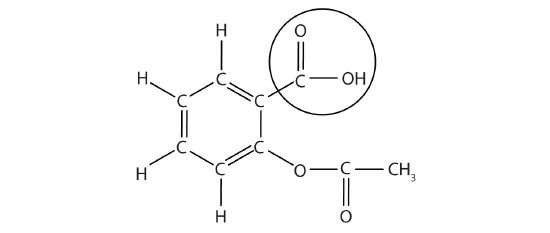
Because the acid properties of aspirin may be problematic, many aspirin brands offer a "buffered aspirin" form of the medicine. In these cases, the aspirin also contains a buffering agent-usually MgO-that regulates the acidity of the aspirin to minimize its acidic side effects.
As useful and common as aspirin is, it was formally marketed as a drug starting in 1899. The US Food and Drug Administration (FDA), the governmental agency charged with overseeing and approving drugs in the United States, wasn't formed until 1906. Some have argued that if the FDA had been formed before aspirin was introduced, aspirin may never have gotten approval due to its potential for side effects-gastrointestinal bleeding, ringing in the ears, Reye's syndrome (a liver problem), and some allergic reactions. However, recently aspirin has been touted for its effects in lessening heart attacks and strokes, so it is likely that aspirin is here to stay.
Buffer solutions are essential components of all living organisms.
- Our blood is buffered to maintain a pH of 7.4 that must remain unchanged as metabolically-generated CO2 (carbonic acid) is added and then removed by our lungs.
- Buffers in the oceans, in natural waters such as lakes and streams, and within soils help maintain their environmental stability against acid rain and increases in atmospheric CO2.
- Many industrial processes, such as brewing, require buffer control, as do research studies in biochemistry and physiology that involve enzymes, are active only within certain pH ranges.
The pH in living systems (Figure \(\PageIndex{1}) is maintained by buffer systems.
| Compartment | pH |
|---|---|
| Gastric Acid | 1 |
| Lysosomes | 4.5 |
| Granules of Chromaffin Cells | 5.5 |
| Human Skin | 5.5 |
| Urine | 6 |
| Neutral H2O at 37 °C | 6.81 |
| Cytosol | 7.2 |
| Cerebrospinal Fluid | 7.3 |
| Blood | 7.43-7.45 |
| Mitochondrial Matrix | 7.5 |
| Pancreas Secretions | 8.1 |
Human blood has a buffering system to minimize extreme changes in pH. One buffer in blood is based on the presence of HCO3− and H2CO3 [H2CO3 is another way to write CO2(aq)]. With this buffer present, even if some stomach acid were to find its way directly into the bloodstream, the change in the pH of blood would be minimal. Inside many of the body’s cells, there is a buffering system based on phosphate ions.
The normal pH of human blood is about 7.4. The carbonate buffer system in the blood uses the following equilibrium reaction:
\[\ce{CO2}(g)+\ce{2H2O}(l)⇌\ce{H2CO3}(aq)⇌\ce{HCO3-}(aq)+\ce{H3O+}(aq) \nonumber \]
The concentration of carbonic acid, H2CO3 is approximately 0.0012 M, and the concentration of the hydrogen carbonate ion, \(\ce{HCO3-}\), is around 0.024 M. Using the Henderson-Hasselbalch equation and the pKa of carbonic acid at body temperature, we can calculate the pH of blood:
\[\mathrm{pH=p\mathit{K}_a+\log\dfrac{[base]}{[acid]}=6.1+\log\dfrac{0.024}{0.0012}=7.4} \nonumber \]
The fact that the H2CO3 concentration is significantly lower than that of the \(\ce{HCO3-}\) ion may seem unusual, but this imbalance is due to the fact that most of the by-products of our metabolism that enter our bloodstream are acidic. Therefore, there must be a larger proportion of base than acid, so that the capacity of the buffer will not be exceeded.
Lactic acid is produced in our muscles when we exercise. As the lactic acid enters the bloodstream, it is neutralized by the \(\ce{HCO3-}\) ion, producing H2CO3. An enzyme then accelerates the breakdown of the excess carbonic acid to carbon dioxide and water, which can be eliminated by breathing. In fact, in addition to the regulating effects of the carbonate buffering system on the pH of blood, the body uses breathing to regulate blood pH. If the pH of the blood decreases too far, an increase in breathing removes CO2 from the blood through the lungs driving the equilibrium reaction such that [H3O+] is lowered. If the blood is too alkaline, a lower breath rate increases CO2 concentration in the blood, driving the equilibrium reaction the other way, increasing [H+] and restoring an appropriate pH.
At this point in this text, you should have the idea that the chemistry of blood is fairly complex. Because of this, people who work with blood must be specially trained to work with it properly.
A blood bank technology specialist is trained to perform routine and special tests on blood samples from blood banks or transfusion centers. This specialist measures the pH of blood, types it (according to the blood’s ABO+/− type, Rh factors, and other typing schemes), tests it for the presence or absence of various diseases, and uses the blood to determine if a patient has any of several medical problems, such as anemia. A blood bank technology specialist may also interview and prepare donors to give blood and may actually collect the blood donation.
Blood bank technology specialists are well trained. Typically, they require a college degree with at least a year of special training in blood biology and chemistry. In the United States, training must conform to standards established by the American Association of Blood Banks.
Key Takeaway
- A buffer is a solution that resists sudden changes in pH.
Concept Review Exercise
- Explain how a buffer prevents large changes in pH.
Answer
- A buffer has components that react with both strong acids and strong bases to resist sudden changes in pH.
Exercises
- Describe a buffer. What two related chemical components are required to make a buffer?
- Can a buffer be made by combining a strong acid with a strong base? Why or why not?
- Which solute combinations can make a buffer? Assume all are aqueous solutions.
- HCl and NaCl
- HNO2 and NaNO2
- NH4NO3 and HNO3
- NH4NO3 and NH3
- Which solute combinations can make a buffer? Assume all are aqueous solutions.
- H3PO4 and Na3PO4
- NaHCO3 and Na2CO3
- NaNO3 and Ca(NO3)2
- HN3 and NH3
- For each combination in Exercise 3 that is a buffer, write the chemical equations for the reactions of the buffer components when a strong acid and a strong base is added.
- For each combination in Exercise 4 that is a buffer, write the chemical equations for the reaction of the buffer components when a strong acid and a strong base is added.
- The complete phosphate buffer system is based on four substances: H3PO4, H2PO4−, HPO42−, and PO43−. What different buffer solutions can be made from these substances?
- Explain why NaBr cannot be a component in either an acidic or a basic buffer.
- Explain why Mg(NO3)2 cannot be a component in either an acidic or a basic buffer.
Answers
- A buffer resists sudden changes in pH. It has a weak acid or base and a salt of that weak acid or base.
- No. Combining a strong acid and a strong base will produce salt and water. Excess strong acid or strong base will not act as a buffer.
-
- not a buffer
- buffer
- not a buffer
- buffer
4. 1. not a buffer
2. buffer
3. not a buffer
4. not buffer
- 3b: strong acid: H+ + NO2− → HNO2; strong base: OH− + HNO2 → H2O + NO2−; 3d: strong acid: H+ + NH3 → NH4+; strong base: OH− + NH4+ → H2O + NH3
- 4b: strong acid: H+ + CO32− → HCO3−; strong base: OH− + HCO3− → H2O + CO32−;
- Buffers can be made by combining H3PO4 and H2PO4−, H2PO4− and HPO42−, and HPO42− and PO43−.
- NaBr splits up into two ions in solution, Na+ and Br−. Na+ will not react with any added base knowing that NaOH is a strong base. Br- will not react with any added acid knowing that HBr is a strong acid. Because NaBr will not react with any added base or acid, it does not resist change in pH and is not a buffer.
- Mg(NO3)2 includes two types of ions, Mg2+ and NO3−. Mg(OH)2 is strong base and completely dissociates (100% falls apart), so Mg2+ will not react with any added base (0% combines with OH−). HNO3 is strong acid and completely dissociates (100% falls apart), so NO3− will not react with any added acid (0% combines with H+). Because Mg(NO3)2 will not react with any added base or acid, it does not resist change in pH and is not a buffer.

