9.6: Epoxide reactions
- Page ID
- 225813
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Epoxide structure
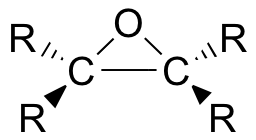
Epoxides (also known as oxiranes) are three-membered ring structures in which one of the vertices is an oxygen and the other two are carbons.
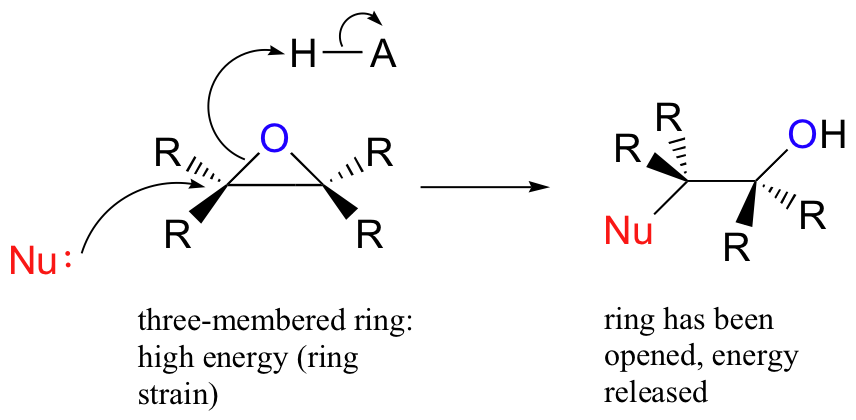
The carbons in an epoxide group are very reactive electrophiles, due in large part to the fact that substantial ring strain is relieved when the ring opens upon nucleophilic attack.
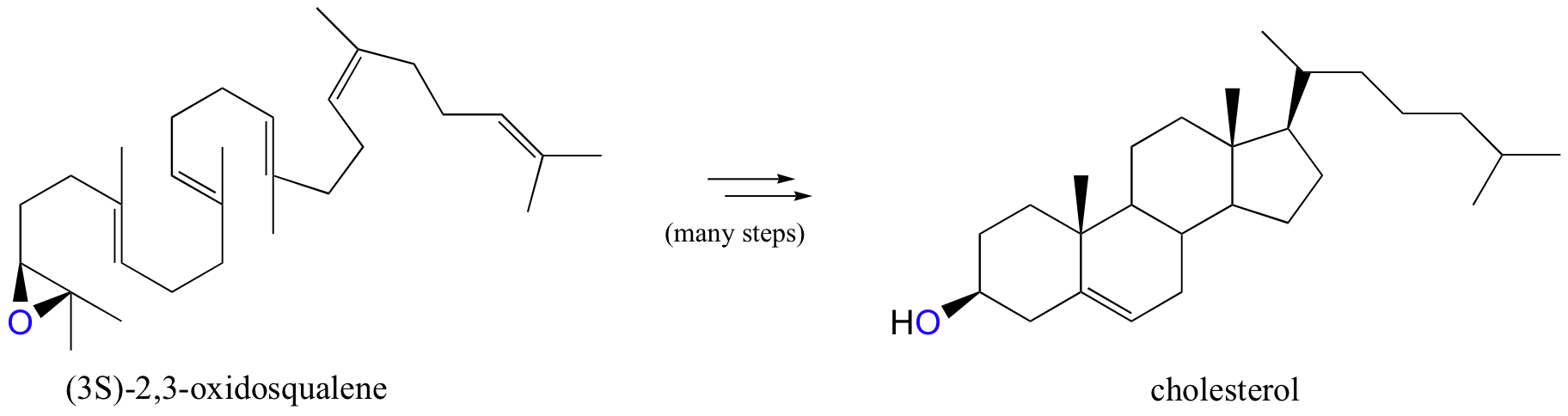
Epoxides are very important intermediates in laboratory organic synthesis, and are also found as intermediate products in some biosynthetic pathways. The compound (3S)-2,3-oxidosqualene, for example, is an important intermediate in the biosynthesis of cholesterol (we’ll see the epoxide ring-opening step in chapter 15):
Both in the laboratory and in the cell, epoxides are usually formed by the oxidation of an alkene. This process will be discussed in detail in section 10.7.
Exercise
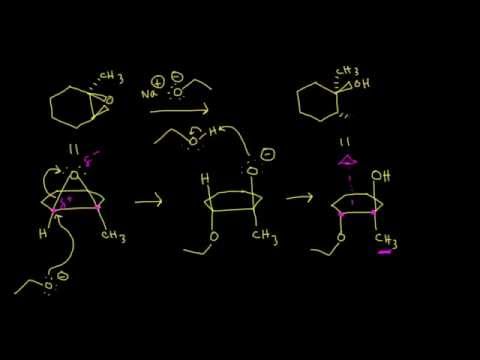
Another way to form an epoxide that does not involve an oxidative reaction is to treat a starting material such as compound A below with a base.
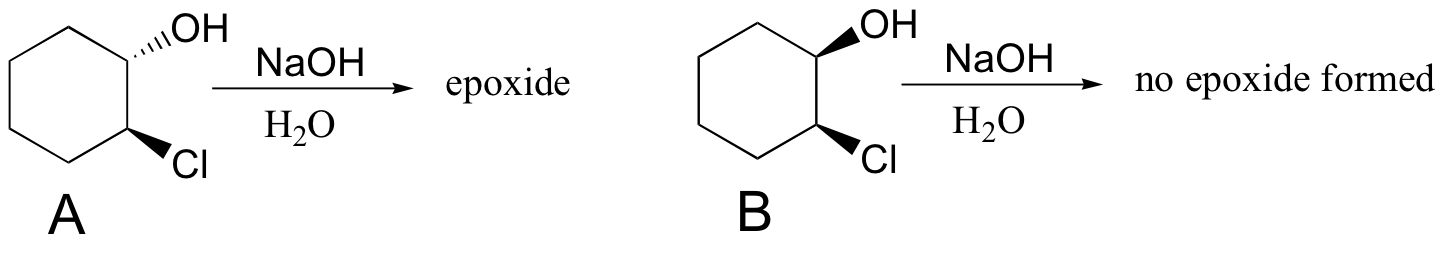
a) Write a mechanism showing the formation of an epoxide from compound A. Show stereochemistry.
b) Compound B does not form an epoxide when treated with base. Why do you think this is, and what does this observation tell you about the mechanism of the epoxide-forming reaction of compound A?
[hidden-answer a=”420009″]a) Notice that the oxygen nucleophile attacks from behind the plane of the page, while the chlorine is pointing out of the plane of the page – this is a backside attack by the nucleophile.
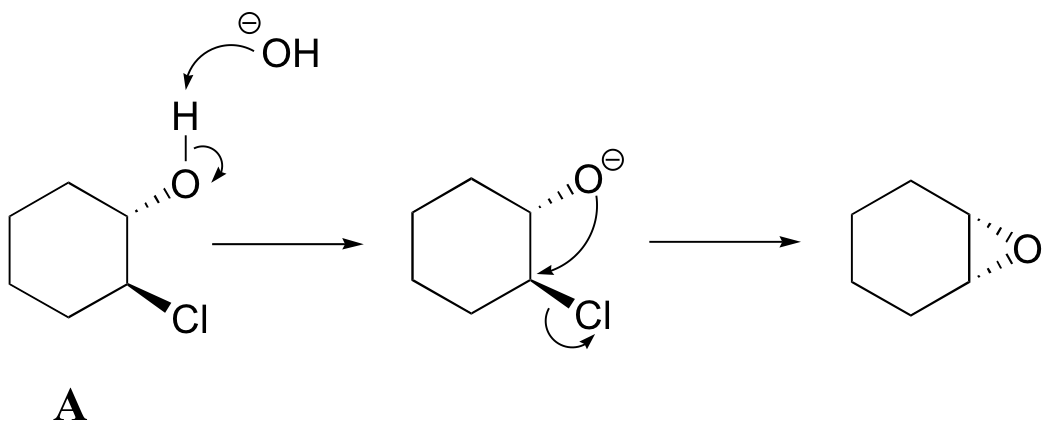
b) Because both the nucleophilic oxygen and the chlorine leaving group are oriented on the same side of the ring, backside attack by the nucleophile is impossible. In order for a displacement to occur, it would have to be a stepwise (SN1) mechanism. The observation that compound A forms an epoxide under these conditions but compound B does not strongly suggests that the reaction proceeds by an SN2 mechanism, with a requirement for backside attack by the nucleophile.[/hidden-answer]
Epoxide ring-opening reactions – SN1 vs. SN2, regioselectivity, and stereoselectivity
The nonenzymatic ring-opening reactions of epoxides provide a nice overview of many of the concepts we have seen already in this chapter. Ring-opening reactions can proceed by either SN2 or SN1 mechanisms, depending on the nature of the epoxide and on the reaction conditions. If the epoxide is asymmetric, the structure of the product will vary according to which mechanism dominates. When an asymmetric epoxide undergoes solvolysis in basic methanol, ring-opening occurs by an SN2 mechanism, and the less substituted carbon is the site of nucleophilic attack, leading to what we will refer to as product B:

Conversely, when solvolysis occurs in acidic methanol, the reaction occurs by a mechanism with substantial SN1 character, and the more substituted carbon is the site of attack. As a result, product A predominates.
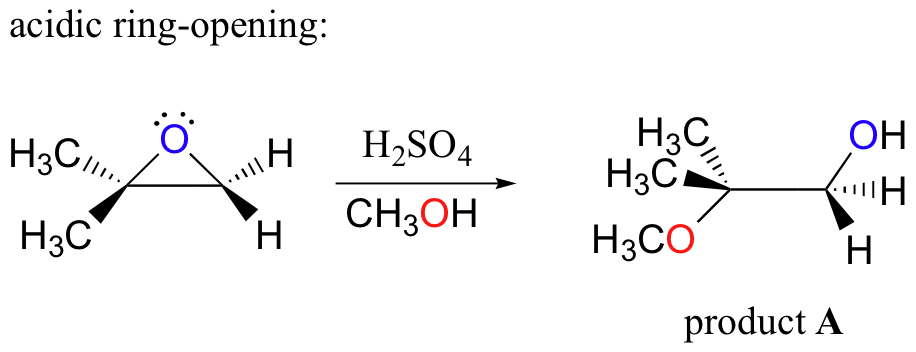
These are both good examples of regioselective reactions. In a regioselective reaction, two (or more) different constitutional isomers are possible as products, but one is formed preferentially (or sometimes exclusively).
Let us examine the basic, SN2 case first. The leaving group is an alkoxide anion, because there is no acid available to protonate the oxygen prior to ring opening. An alkoxide is a poor leaving group, and thus the ring is unlikely to open without a ‘push’ from the nucleophile.
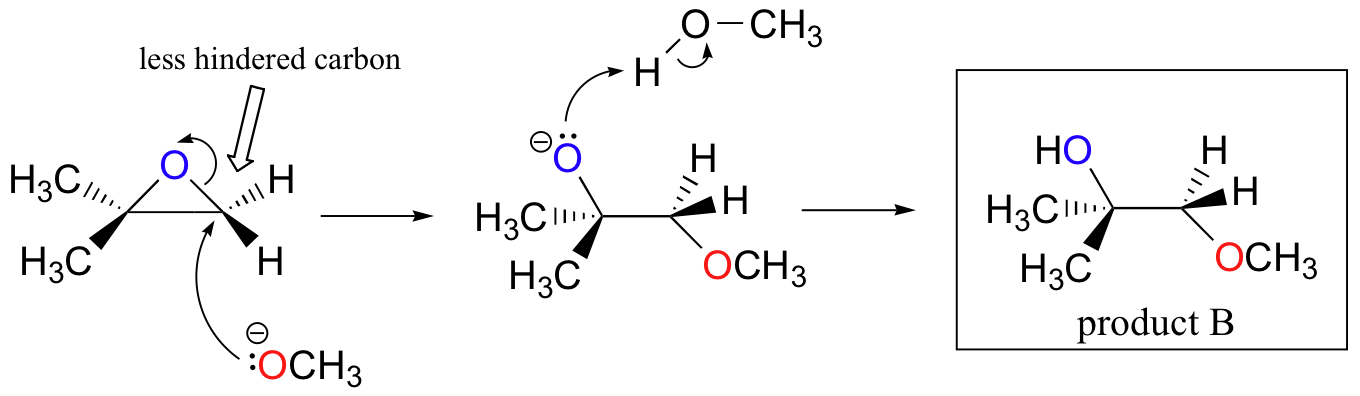
The nucleophile itself is potent: a deprotonated, negatively charged methoxide ion. When a nucleophilic substitution reaction involves a poor leaving group and a powerful nucleophile, it is very likely to proceed by an SN2 mechanism.
What about the electrophile? There are two electrophilic carbons in the epoxide, but the best target for the nucleophile in an SN2 reaction is the carbon that is least hindered. This accounts for the observed regiochemical outcome. Like in other SN2 reactions, nucleophilic attack takes place from the backside, resulting in inversion at the electrophilic carbon.
Probably the best way to depict the acid-catalyzed epoxide ring-opening reaction is as a hybrid, or cross, between an SN2 and SN1 mechanism. First, the oxygen is protonated, creating a good leaving group (step 1 below) . Then the carbon-oxygen bond begins to break (step 2) and positive charge begins to build up on the more substituted carbon (recall the discussion from section 8.4B about carbocation stability).
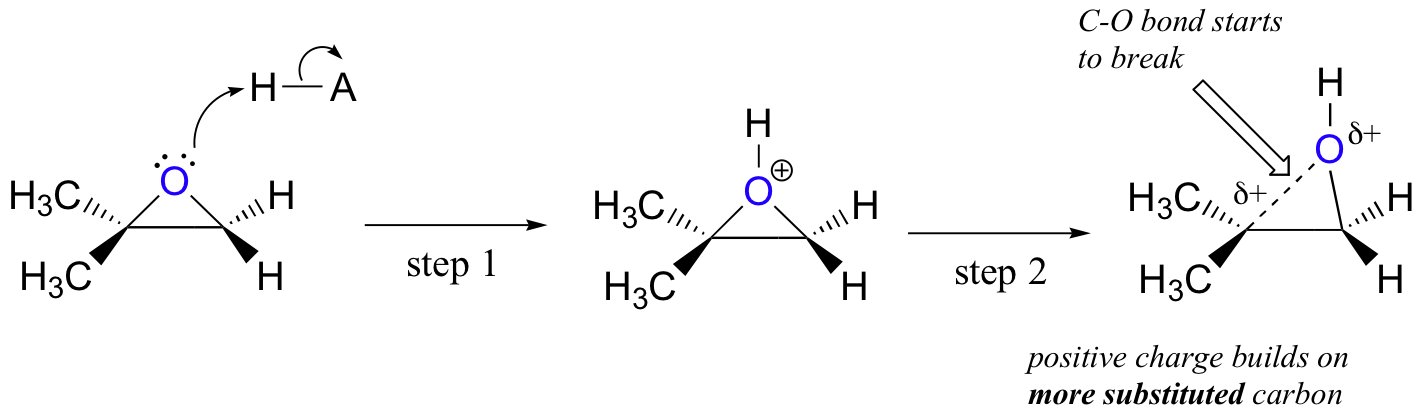
Unlike in an SN1 reaction, the nucleophile attacks the electrophilic carbon (step 3) before a complete carbocation intermediate has a chance to form.
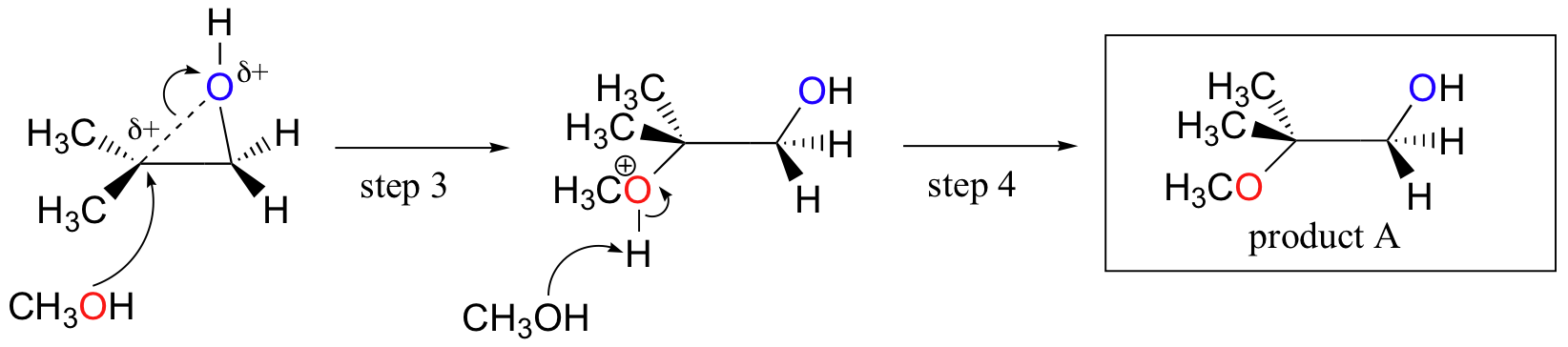
Attack takes place preferentially from the backside (like in an SN2 reaction) because the carbon-oxygen bond is still to some degree in place, and the oxygen blocks attack from the front side. Notice, however, how the regiochemical outcome is different from the base-catalyzed reaction: in the acid-catalyzed process, the nucleophile attacks the more substituted carbon because it is this carbon that holds a greater degree of positive charge.
Exercise 8.17:
Predict the major product(s) of the ring opening reaction that occurs when the epoxide shown below is treated with:
a) ethanol and a small amount of sodium hydroxide
b) ethanol and a small amount of sulfuric acid
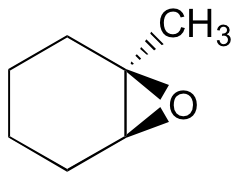
Hint: be sure to consider both regiochemistry and stereochemistry!
[hidden-answer a=”823600″]
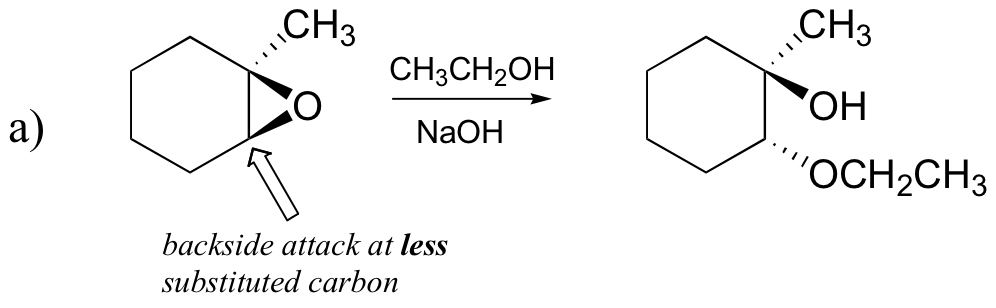
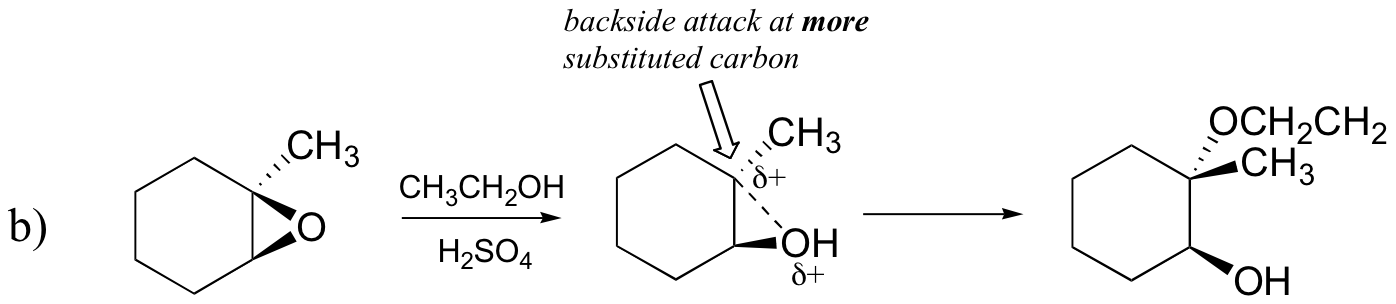 [/hidden-answer]
[/hidden-answer]Khan academy videos

Ring opening under acidic conditions:

- 8.6: Epoxides as electrophiles in nucleophilic substitution reactions. Authored by: u00a0Tim Soderbergu00a0(University of Minnesota, Morris). Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg)/Chapter_08%3A_Nucleophilic_substitution_reactions_I/8.6%3A_Epoxides_as_electrophiles_in_nucleophilic_substitution_reactions. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike