19.7: Oxidation of alkenes
- Page ID
- 225886
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
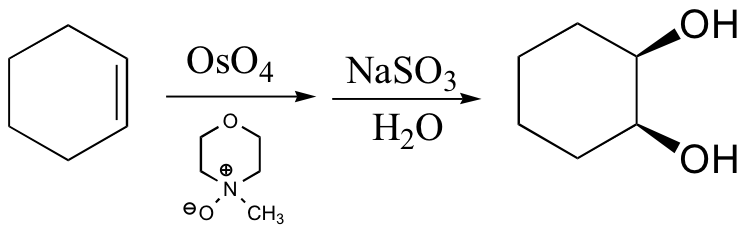
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Dihydroxylation of alkenes
Examples
Questions:
1. Give the major product.
.bmp?revision=1&size=bestfit&width=131&height=51)
2. What is the product in the dihydroxylation of (Z)-3-hexene?

3. What is the product in the dihydroxylation of (E)-3-hexene?

4. Draw the intermediate of this reaction.

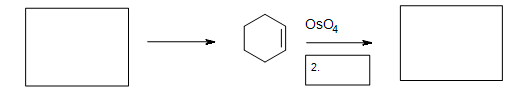
5. Fill in the missing reactants, reagents, and product.
Solutions
[reveal-answer q=”18923″]Show Answer[/reveal-answer]
[hidden-answer a=”18923″]
1. A syn-1,2-ethanediol is formed. There is no stereocenter in this particular reaction. The OH groups are on the same side.
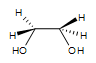
2. Meso-3,4-hexanediol is formed. There are 2 stereocenters in this reaction.
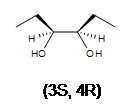
3. A racemic mixture of 3,4-hexanediol is formed. There are 2 stereocenters in both products.

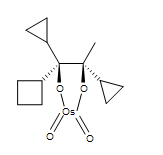
4. A cyclic osmic ester is formed.
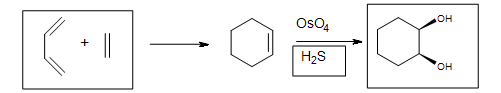
5. The Diels-Alder cycloaddition reaction is needed in the first box to form the cyclohexene. The second box needs a reagent to reduce the intermediate cyclic ester (not shown). The third box has the product: 1,2-cyclohexanediol.
 [/hidden-answer]
[/hidden-answer]
Epoxidation of alkenes
Alkenes can be oxidized to epoxides using a ‘peroxyacid‘ such as m-chloroperoxybenzoic acid (MCPBA). Notice the presence of a third oxygen in the peroxyacid functional group.
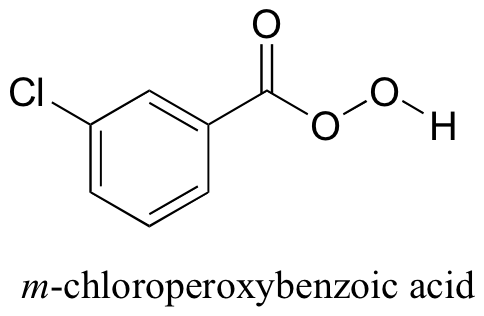
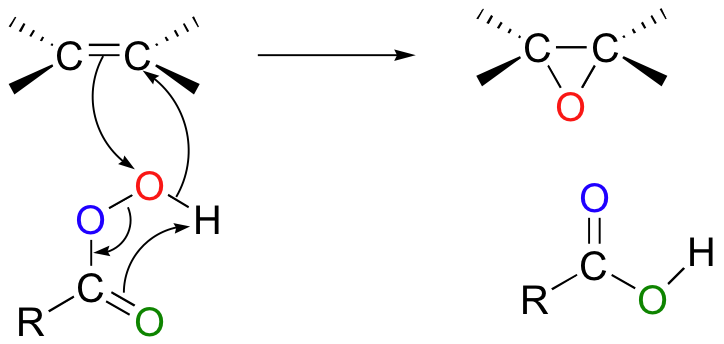
The mechanism was covered earlier in section 10.7. The π electrons in the alkene double bond attacking the ‘outer’ oxygen of the peroxyacid and cleaving the reactive O-O peroxide bond.
Uncatalyzed epoxidation of an asymmetric alkene generally results in two diastereomeric epoxide products, with the epoxide adding either from above or below the plane of the alkene.
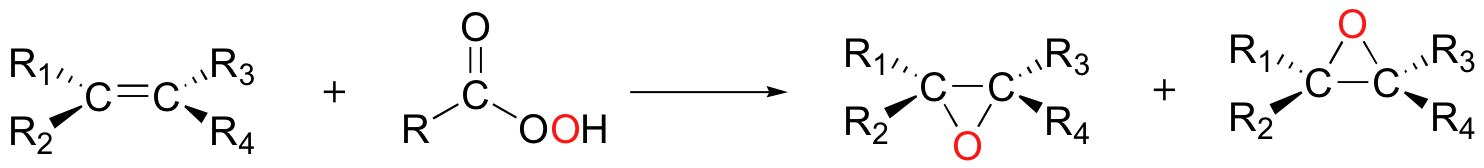
Epoxides are very useful intermediates in organic synthesis, as we learnt in section 9.6.
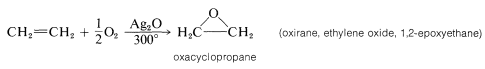
A reaction of immense industrial importance is the formation of oxacyclopropane itself (most often called ethylene oxide) by oxidation of ethene with oxygen over a silver oxide catalyst at $$300^\text{o}$$:
Oxacyclopropane (ethylene oxide) is used for many purposes, but probably the most important reaction is ring opening with water to give 1,2-ethanediol (ethylene glycol, bp $$197^\text{o}$$). This diol, mixed with water, is employed widely in automotive cooling systems to provide both a higher boiling and lower freezing coolant than water alone:
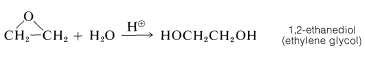
External links
Carey 5th Ed Online, Epoxidation of Alkenes
Exercises
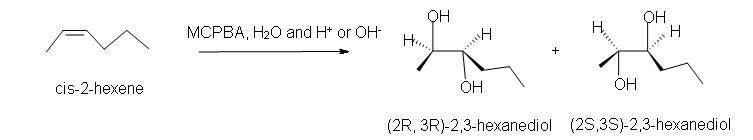
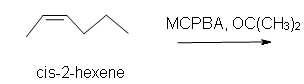
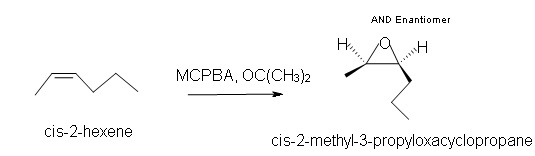
1. Predict the product of the reaction of cis-2-hexene with MCPBA (meta-chloroperoxybenzoic acid)
a) in acetone solvent.
b) in an aqueous medium with acid or base catalyst present.
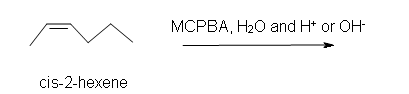
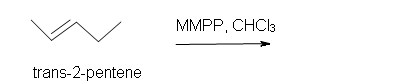
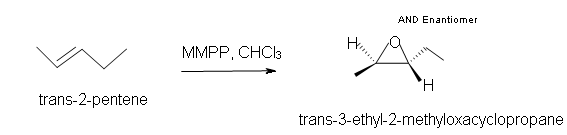
2. Predict the product of the reaction of trans-2-pentene with magnesium monoperoxyphthalate (MMPP) in a chloroform solvent.
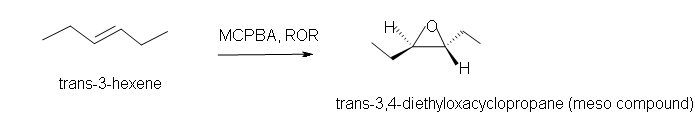
3. Predict the product of the reaction of trans-3-hexene with MCPBA in ether solvent.

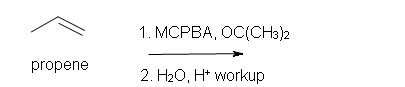
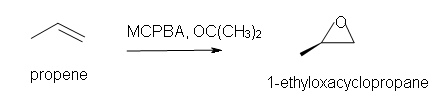
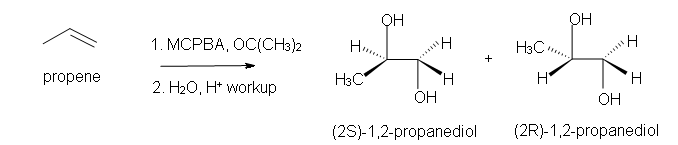
4. Predict the reaction of propene with MCPBA.
a) in acetone solvent
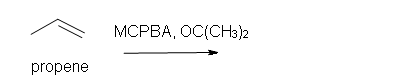
b) after aqueous work-up.
5. Predict the reaction of cis-2-butene in chloroform solvent.
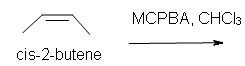
Answers
[reveal-answer q=”935928″]Show Answer[/reveal-answer]
[hidden-answer a=”935928″]
1. a) Cis-2-methyl-3-propyloxacyclopropane
b) Racemic (2R,3R)-2,3-hexanediol and (2S,3S)-2,3-hexanediol
2. Trans-3-ethyl-2-methyloxacyclopropane.
3. Trans-3,4-diethyloxacyclopropane.
4. a) 1-ethyl-oxacyclopropane
b) Racemic (2S)-1,2-propandiol and (2R)-1,2-propanediol
5. Cis-2,3-dimethyloxacyclopropane
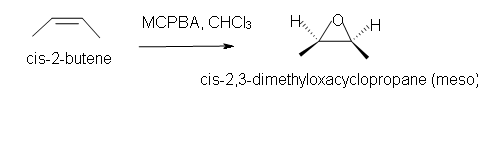 [/hidden-answer]
[/hidden-answer]
Contributors
- Kristen Perano
Oxidative cleavage
With oxidative cleavage, the carbon-carbon bond of an alkene is completely broken, and in many cases this will break the molecule into two pieces. Alkenes can be cleaved by oxidation with ozone, O3., using a process called ozonolysis. The carbon-carbon double bond is broken, and the alkene carbons are converted to aldehydes:
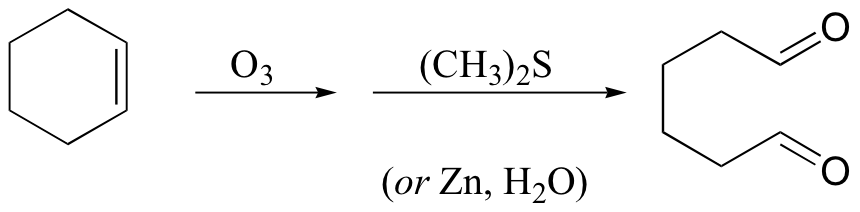
Dimethyl sulfide or zinc is added in the work-up stage of the reaction in order to reduce hydrogen peroxide, which is formed in the reaction, to water.
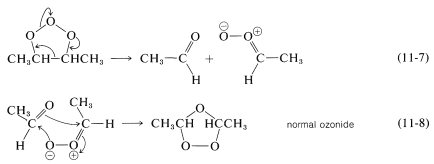
The simplest and most widely accepted mechanism for ozonolysis involves formation of a molozonide by a direct cycloaddition of ozone to the double bond.$$^1$$

Isomerization of the molozonide appears to occur by a fragmentation-recombination reaction, as shown in Equations 11-7 and 11-8:
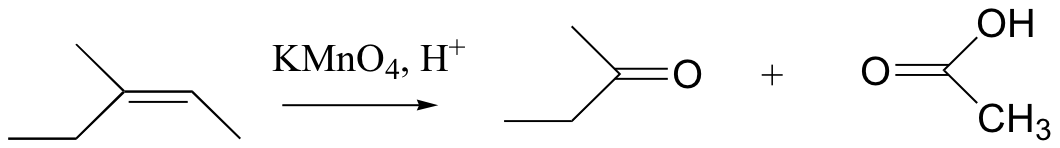
Potassium permanganate (KMnO4) is another very powerful oxidizing agent that will oxidize primary alcohols and aldehydes to carboxylic acids. KMnO4 is also useful for oxidative cleavage of alkenes to ketones and carboxylic acids:
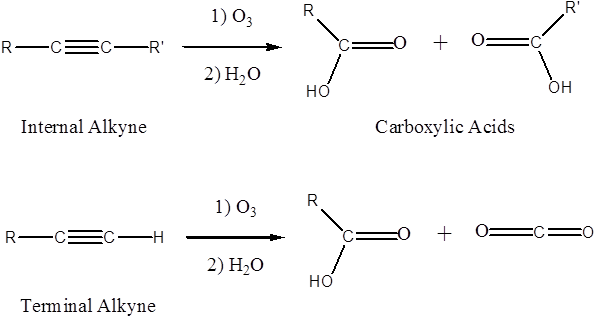
Alkynes can also undergo oxidative cleavage. Internal alkynes form carboxylic acids (RCOOH) and terminal alkynes form carboxylic acids and CO2. The ozonide intermediate only requires water to decompose it to the cleavage products:
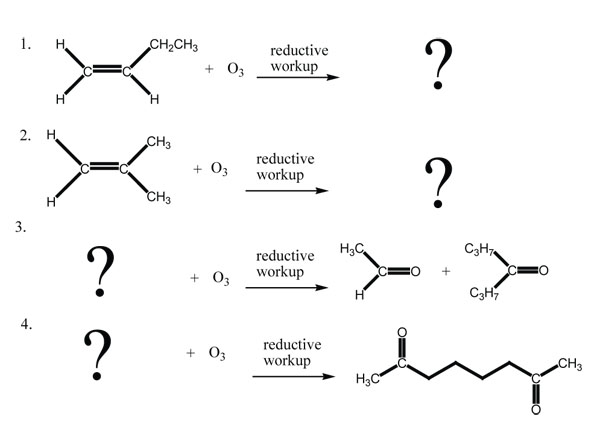
Exercises
Answers
[reveal-answer q=”497037″]Show Answer[/reveal-answer]
[hidden-answer a=”497037″]
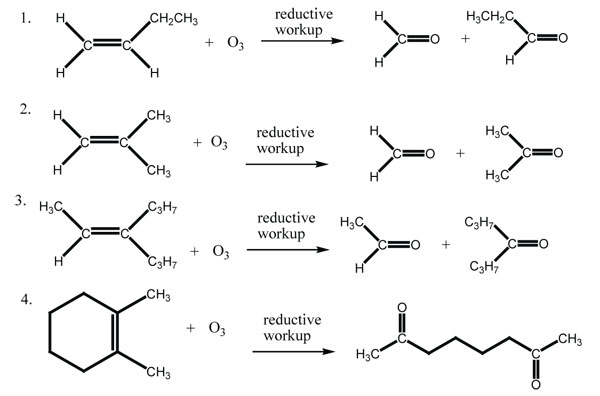 [/hidden-answer]
[/hidden-answer]
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- John D. Robert and Marjorie C.Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, “You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format.”
Video

- Organic Chemistry With a Biological Emphasis . Authored by: Tim Soderberg. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_12%3A_Oxidation_and_Reduction/12.07_Oxidizing_Agents. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 11.3.6 Epoxidation of Alkenes. Authored by: Kristen Perano. Located at: https://chem.libretexts.org/LibreTexts/Purdue/Purdue%3A_Chem_26605%3A_Organic_Chemistry_II_(Lipton)/Chapter_11.__Addition_to_pi_Systems/11.3%3A_Concerted_Additions/11.3.6_Epoxidation_of_Alkenes. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 12.11: Vicinal SYn Dihydroxylation with Osmium Tetroxide. Authored by: Dr. Dietmar Kennepohl, Prof. Steve Farmer, Shivam Nand, Kristen Perano. Located at: https://chem.libretexts.org/LibreTexts/Winona_State_University/Klein_and_Straumanis_Guided/9%3A_Addition_Reactions_of_Alkenes/12.11%3A_Vicinal__SYn_Dihydroxylation_with__Osmium_Tetroxide. Project: Chemistry LibreText. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Ozonolysis. Authored by: Prof. Steve Farmer. Located at: https://chem.libretexts.org/LibreTexts/Purdue/Purdue_Chem_26100%3A_Organic_Chemistry_I_(Wenthold)/Chapter_09%3A_Alkynes/9.5_Oxidation_and_Reduction_of_Alkynes/9.5.3._Permanganate_and_Ozonolysis/Ozonolysis. Project: Chemistry Libretexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike