11.3.6 Epoxidation of Alkenes
- Page ID
- 22909
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Oxacyclopropane rings, also called epoxide rings, are useful reagents that may be opened by further reaction to form anti vicinal diols. One way to synthesize oxacyclopropane rings is through the reaction of an alkene with peroxycarboxylic acid.
Oxacyclopropane Synthesis by Peroxycarboxylic Acid
Oxacyclopropane synthesis by peroxycarboxylic acid requires an alkene and a peroxycarboxylic acid as well as an appropriate solvent. The peroxycarboxylic acid has the unique property of having an electropositive oxygen atom on the COOH group. The reaction is initiated by the electrophilic oxygen atom reacting with the nucleophilic carbon-carbon double bond. The mechanism involves a concerted reaction with a four-part, circular transition state. The result is that the originally electropositive oxygen atom ends up in the oxacyclopropane ring and the COOH group becomes COH.
Mechanism
Peroxycarboxylic acids are generally unstable. An exception is meta-chloroperoxybenzoic acid, shown in the mechanism above. Often abbreviated MCPBA, it is a stable crystalline solid. Consequently, MCPBA is popular for laboratory use. However, MCPBA can be explosive under some conditions.
.png?revision=1)
Peroxycarboxylic acids are sometimes replaced in industrial applications by monoperphthalic acid, or the monoperoxyphthalate ion bound to magnesium, which gives magnesium monoperoxyphthalate (MMPP). In either case, a nonaqueous solvent such as chloroform, ether, acetone, or dioxane is used. This is because in an aqueous medium with any acid or base catalyst present, the epoxide ring is hydrolyzed to form a vicinal diol, a molecule with two OH groups on neighboring carbons. (For more explanation of how this reaction leads to vicinal diols, see below.) However, in a nonaqueous solvent, the hydrolysis is prevented and the epoxide ring can be isolated as the product. Reaction yields from this reaction are usually about 75%. The reaction rate is affected by the nature of the alkene, with more nucleophilic double bonds resulting in faster reactions.
| Example |
|---|
|
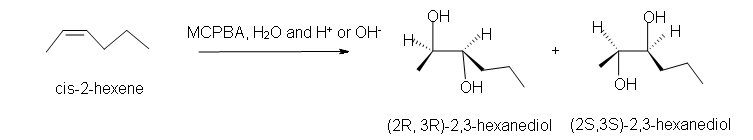
Since the transfer of oxygen is to the same side of the double bond, the resulting oxacyclopropane ring will have the same stereochemistry as the starting alkene. A good way to think of this is that the alkene is rotated so that some constituents are coming forward and some are behind. Then, the oxygen is inserted on top. (See the product of the above reaction.) One way the epoxide ring can be opened is by an acid catalyzed oxidation-hydrolysis. Oxidation-hydrolysis gives a vicinal diol, a molecule with OH groups on neighboring carbons. For this reaction, the dihydroxylation is anti since, due to steric hindrance, the ring is attacked from the side opposite the existing oxygen atom. Thus, if the starting alkene is trans, the resulting vicinal diol will have one S and one R stereocenter. But, if the starting alkene is cis, the resulting vicinal diol will have a racemic mixture of S, S and R, R enantiomers. |
References
- Royals, E. 1954. Advanced Organic Chemistry. New York: Prentice Hall. 948 p.
- Streitwieser, A. and C. Heathcock. 1981. Introduction to Organic Chemistry. 2nd ed. New York: Macmillan Publishing Co. 1258 p.
- Vollhardt, K. and N. Schore. 2007. Organic Chemistry: Structure and Function. 5th ed. New York: W.H. Freeman and Company. 1254 p.
- Wheland, G. 1949. Advanced Organic Chemistry. 3rd ed. New York: John Wiley & Sons. 871 p.
Outside Links
Problems
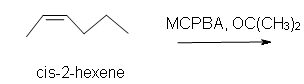
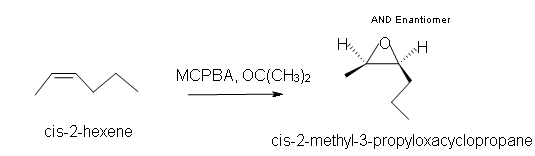
1. Predict the product of the reaction of cis-2-hexene with MCPBA (meta-chloroperoxybenzoic acid)
a) in acetone solvent.
b) in an aqueous medium with acid or base catalyst present.
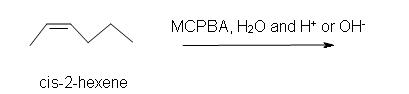
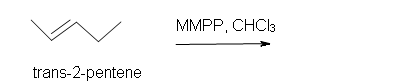
2. Predict the product of the reaction of trans-2-pentene with magnesium monoperoxyphthalate (MMPP) in a chloroform solvent.
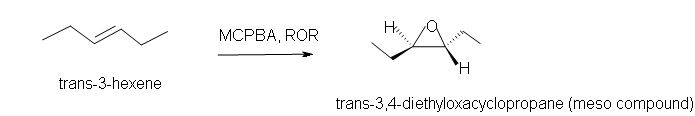
3. Predict the product of the reaction of trans-3-hexene with MCPBA in ether solvent.

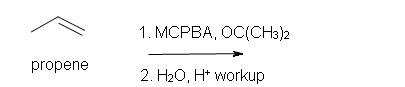
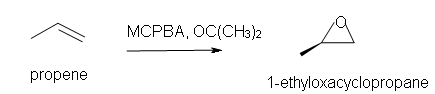
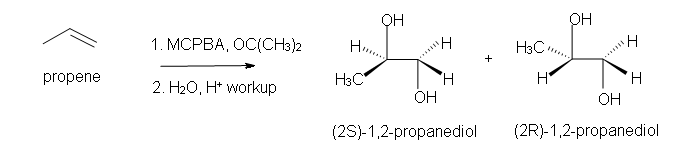
4. Predict the reaction of propene with MCPBA.
a) in acetone solvent
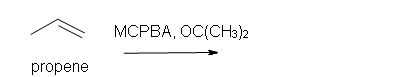
b) after aqueous work-up.
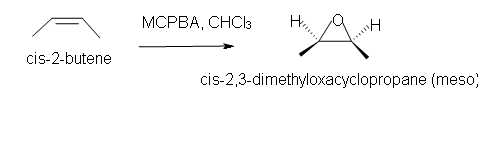
5. Predict the reaction of cis-2-butene in chloroform solvent.
.png?revision=1&size=bestfit&width=245&height=66)
Answers
1. a) Cis-2-methyl-3-propyloxacyclopropane
b) Racemic (2R,3R)-2,3-hexanediol and (2S,3S)-2,3-hexanediol
2. Trans-3-ethyl-2-methyloxacyclopropane.

3. Trans-3,4-diethyloxacyclopropane.
4. a) 1-ethyl-oxacyclopropane
b) Racemic (2S)-1,2-propandiol and (2R)-1,2-propanediol
5. Cis-2,3-dimethyloxacyclopropane
Contributors
- Kristen Perano
Further Reading
Carey 5th Ed Online
Youtube
Leah4Sci
Chemtube3D


.png?revision=1&size=bestfit&width=686&height=193)