17.2: Palladium catalyzed couplings
- Page ID
- 225873
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
In this lecture you will learn the following:
- The C−C cross-coupling reactions and their mechanisms.
The palladium catalyzed cross-coupling reactions are a class of highly successful reactions with applications in the organic synthesis to have emerged recently. The reactions carry out a coupling of the aryl, vinyl or alkyl halide substrates with different organometallic nucleophiles and as such encompasses a family of C−C cross-coupling reactions that are dependent on the nature of nucleophiles like that of the B based ones in the Suzuki-Miyuara coupling, the Sn based ones in the Stille coupling, the Si based ones in the Hiyama coupling, the Zn based ones in the Negishi coupling and the Mg based ones in the Kumada coupling reactions (Figure 1).
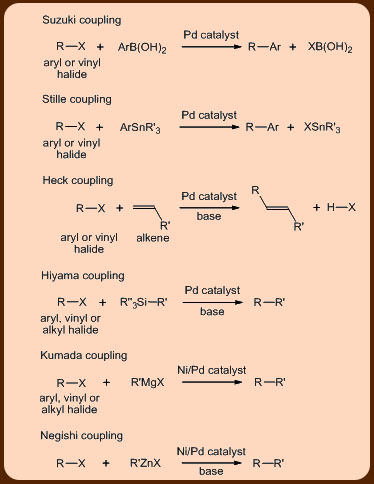
An unique feature of these reactions is the exclusive formation of the cross-coupled product without the accompaniment of any homo-coupled product (i.e., where the aryl halide couples with itself). Another interesting feature of these coupling reactions is that they proceed via a common mechanism involving three steps that include the oxidative addition, the transmetallation and the reductive elimination reactions (Figures 2 and 3).
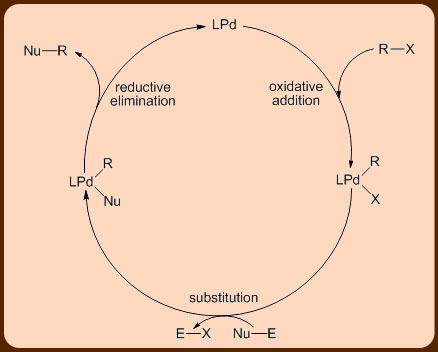
Summary
Organometallic complexes play a pivotal role in several successful homogeneous catalysis reactions like that of the hydroformylation and the C−C cross-coupling reactions. These reactions are important because of the fact that both of the hydroformylation and the C−C cross-coupling reactions give more value added products compared to the starting reactants. The palladium catalyzed C−C cross-coupling reactions are a class of highly successful reactions that have permanently impacted the area of organic synthesis in a profound way to an extent that the 2010 Nobel prize has been conferred on one of these reactions thereby recognizing the importance of the C−C cross-coupling recations.
Contributors
http://nptel.ac.in/courses/104101006/31
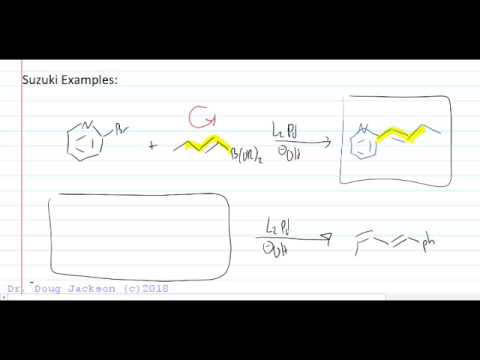
Suzuki-Miyaura coupling (or Suzuki coupling) is a metal catalyzed reaction, typically with Pd, between an alkenyl (vinyl), aryl, or alkynyl organoborane (boronic acid or boronic ester, or special cases with aryl trifluoroborane) and halide or triflate under basic conditions. This reaction is used to create carbon-carbon bonds to produce conjugated systems of alkenes, styrenes, or biaryl compounds (Scheme 1).
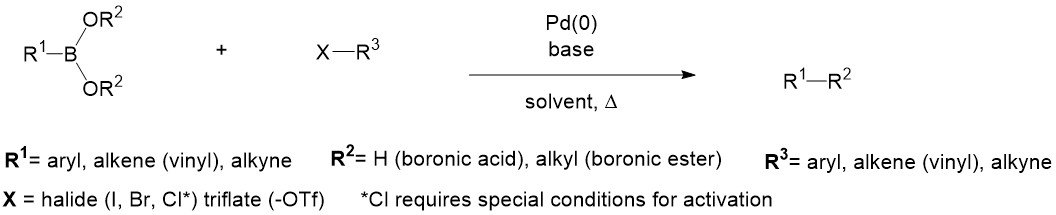
Modified conditions have demonstrated reactivity with less reactive substrates such as alkyl boranes (BR3) or aryl or alkenyl chlorides by amending the base and ligands employed.
Historical Methods: Forming carbon-carbon bonds via cross coupling reactions
The Suzuki coupling is a pioneering reaction in cross coupling, and has been thoroughly studied since. The first Suzuki-type cross coupling reaction between phenylboronic acid and haloarenes was published by Suzuki and Miyaura in 1981 (Scheme 1).1 Commonly, Suzuki coupling is compared to Stille coupling seeing that boron has a similar electronegativity to tin, which is used for transmetallation in Stille coupling. Both couplings have a similar reaction scope and proceed via a similar mechanistic cycle.
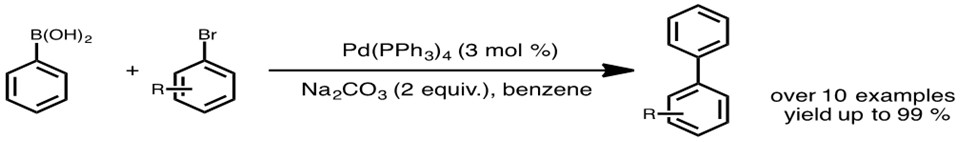
Video

Reference
- (1) Miyaura, N.; Suzuki, A. Chem. Rev., 1995, 95, 2457.
Catalysts
Palladium catalysts are most widely employed in Suzuki coupling. Normally, the active Pd catalyst consist of two parts: precursors (for example: Pd(OAc)2, Pd2(dba)3, or Pd(PPh3)4), and ligands.
The order of reactivity of the halogen leaving groups is as follows:
I >> Br > OTf >> Cl > F
Advantages of Suzuki Coupling
Typically, cross coupling reactions are run in organic conditions; however, Suzuki couplings can be performed in heterogeneous or purely aqueous conditions as organoboranes are water soluble and compatible with water soluble, inorganically supported, ligand-free Pd-catalysts. Boranes are exceptionally nucleophilic, and thus do not require extreme conditions for transmetallation which increases its functional group tolerance. Their high reactivity is also demonstrated in high turnover rates of the Pd catalyst used, which allows for a lower loading.
Organoboranes are nontoxic and stable to extreme heating and exposure to oxygen or water. Consequently, these reagents can be easily used at benchtop, and do not require special equipment or techniques, such as gloveboxes or air-sensitive and dry technique.1 This reaction has a high atom economy seeing that the byproducts are typically salts and water soluble borane byproducts.1.4 The lack of interfering byproducts allows for one-pot syntheses to further reduce waste and increase reaction efficiency.4 These attractive features of organoborane reagents increases its utility and synthetic convenience, and makes Suzuki coupling optimal for large scale and industry synthesis.
However, at present only a few boronic acids are cheaply available; a larger number are available but expensive.
Reference
- Paul, S.; Islam, M. M.; Islam, S. M. RSC Adv. 2015, 5, 42193.
Contributors
- Peiyuan Qu and Amanda Ramdular
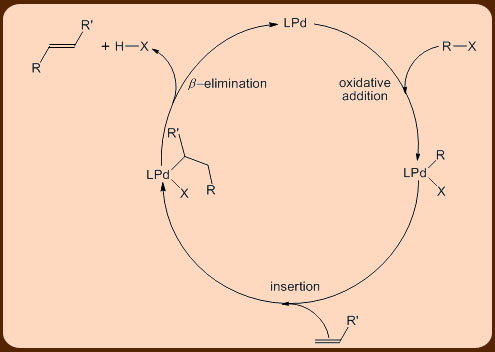
Heck Reaction
The Heck reaction is a famous chemical reaction discovered by Mizoroki and Heck in 1972 through independent research. It involves the cross-coupling reaction between organohalides and alkenes, these two substances react in the presence of a palladium catalyst and a base to form a substituted alkene:
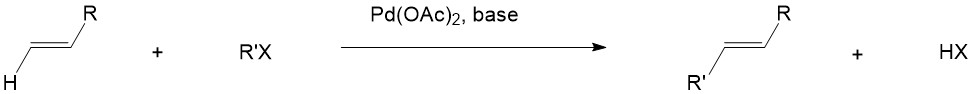
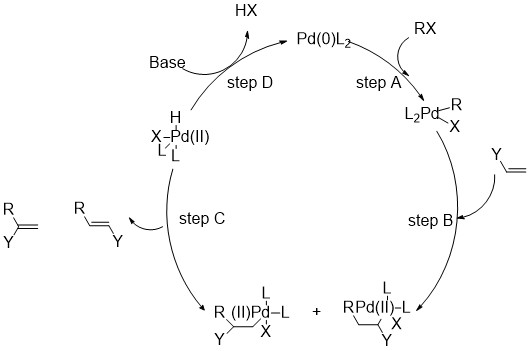
Step A is the oxidative addition of a polar substrate onto a palladium catalyst to form a tetrasubstituted complex. Step B is the migratory insertion of an olefin into the system. Step C is the β -Hydride Elimination of the alkene and Step D is the addition of base to the palladium to regenerate the starting catalyst and close the cycle[1].
Contributors
- Haley Merritt and Yifan Qi
Video

- 25.5F: Cu2212C cross-coupling reactions. Authored by: http://nptel.ac.in/courses/104101006/31. Located at: https://chem.libretexts.org/Textbook_Maps/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_(Housecroft)/25%3A_Catalysis_and_some_industrial_processes/25.5%3A_Homogeneous_Catalysis_-_Industrial_Applications/25.5F%3A_C%E2%88%92C_cross-coupling_reactions. Project: Chemistry LibreTexts. License: Public Domain: No Known Copyright
- Suzuki-Miyaura Coupling. Authored by: Peiyuan Qu and Amanda Ramdular. Located at: https://chem.libretexts.org/Textbook_Maps/Inorganic_Chemistry/Supplemental_Modules_(Inorganic_Chemistry)/Catalysis/Catalyst_Examples/Suzuki-Miyaura_Coupling. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Heck Reaction. Authored by: Haley Merritt and Yifan Qi. Located at: https://chem.libretexts.org/Textbook_Maps/Inorganic_Chemistry/Supplemental_Modules_(Inorganic_Chemistry)/Catalysis/Catalyst_Examples/Heck_Reaction. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike