9.P: Radical Reactions (Problems)
- Page ID
- 291186
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)P16.1: Plexiglass is a polymer of methyl methacrylate. Show a mechanism for the first two propagation steps of polymerization (use \(X\cdot\) to denote the radical initiator), and show a structure for the plexiglass polymer. Assume an alkene addition process similar to that shown in the text for polyethylene.
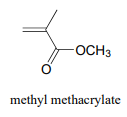
P16.2: In section 16.3 we saw how acrylamide polymerizes to form the polyacrylamide used in PAGE protein gels. Polyacrylamide by itself is not sufficient by itself to form the gel - the long polyacrylamide chains simply slip against each other, like boiled spaghetti. To make a PAGE gel, with pores for the proteins to slip through, we need a crosslinker - something to tie the chains together, forming a three-dimensional web-like structure. Usually, a small amount of bis-acrylamide is added to the acrylamide in the polymerization mixture for this purpose.
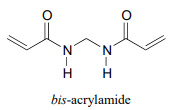
Propose a radical mechanism showing how bis- acrylamide might form crosslinks between two polyacrylamide chains.
P16.3: Resveratrol is a natural antioxidant found in red wine (see section 16.5 for the structure).
- Draw one resonance structure to illustrate how the resveratrol radical is delocalized by resonance.
- Indicate all of the carbons on your structure to which the radical can be delocalized.
- Draw an alternate resveratrol radical (one in which a hydrogen atom from one of the other two phenolic groups has been abstracted). To how many carbons can this radical be delocalized?
- The curcumin structure is shown in the same figure as that of resveratrol, in section16.5. Draw two resonance contributors of a curcumin radical, one in which the unpaired electron is on a phenolic oxygen, and one in which the unpaired electron is on a ketone oxygen.
P16.4: Draw the radical intermediate species that you would expect to form when each of the compounds below reacts with a radical initiator.

P16.5: Azobis(isobutyronitrile) is a widely used radical initiator which rapidly undergoes homolytic decomposition when heated.
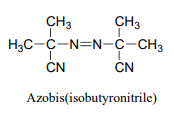
Predict the products of this decomposition reaction, and show a likely mechanism. What is the thermodynamic driving force for homolytic cleavage?
P16.6:
- When 2-methylbutane is subjected to chlorine gas and heat, a number of isomeric chloroalkanes with the formula \(C_5H_{11}Cl\) can form. Draw structures for these isomers, and for each draw the alkyl radical intermediate that led to its formation.
- In part a), which is the most stable radical intermediate?
- In the reaction in part a), the relative abundance of different isomers in the product is not exclusively a reflection of the relative stability of radical intermediates. Explain.
P16.7: We learned in chapter 14 that \(HBr\) will react with alkenes in electrophilic addition reactions with 'Markovnikov' regioselectivity. However, when the starting alkene contains even a small amount of contaminating peroxide (which happens when it is allowed to come into contact with air), a significant amount of 'anti-Markovnikov' product is often observed.
- Propose a mechanism for formation of the anti-Markovnikov addition product when 1-butene reacts with \(HBr\) containing a small amount of benzoyl peroxide
- Predict the product and propose a mechanism for the addition of ethanethiol to 1-butene in the presence of peroxide.
P16.8: In section 11.5 we learned that aspirin works by blocking the action of an enzyme that catalyzes a key step in the biosynthesis of prostaglandins, a class of biochemical signaling molecules. The enzyme in question, prostaglandin \(H\) synthase (EC 1.14.99.1) catalyzes the reaction via several single-electron steps. First, an iron-bound oxygen radical in the enzyme abstracts a hydrogen atom from arachidonate. The arachidonate radical intermediate then reacts with molecular oxygen to form a five-membered oxygen-containing ring, followed by closure of a cyclopentane ring to yield yet another radical intermediate. (Biochemistry 2002, 41, 15451.)
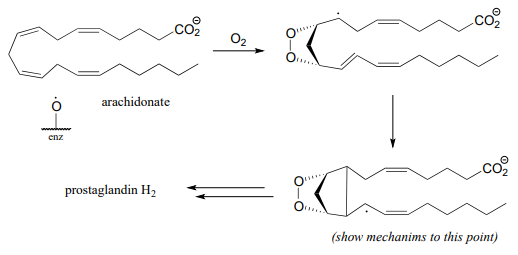
Propose a mechanism for the steps of the reaction that are shown in this figure.
P16.9: Some redox enzymes use copper to assist in electron transfer steps. One important example is dopamine b-monooxygenase (EC 1.14.1.1), which catalyzes the following reaction:
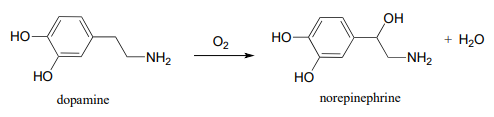
The following intermediates have been proposed: (see Biochemistry 1994, 33, 226) Silverman p. 222
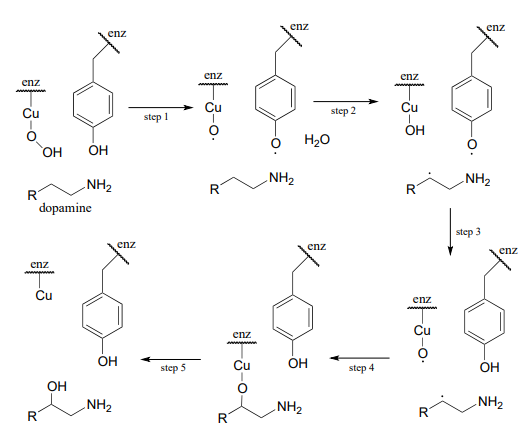
Draw mechanistic arrows for steps 1-4.
Contributors
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


