1.4: ATP, The Principal Phosphate Group Donor
- Page ID
- 291116
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Thus far we have been very general in our discussion of phosphate transfer reactions, referring only to generic 'donor' and 'acceptor' species. It's time to get more specific. The most important donor of phosphate groups in the cell is a molecule called adenosine triphosphate, commonly known by its abbreviation ATP.
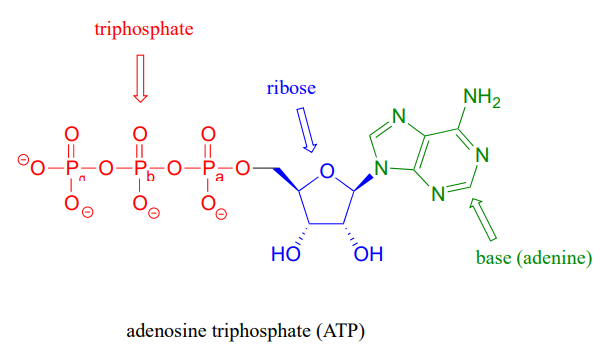
that there are essentially three parts to the ATP molecule: an adenine nucleoside 'base', a five-carbon sugar (ribose), and triphosphate. The three phosphates are designated by Greek letters a, b, and g, with the a phosphate being the one closest to the ribose. Adenosine diphosphate (ADP) and adenosine monophosphate (AMP) are also important players in the reactions of this chapter.
ATP is a big molecule, but the bond-breaking and bond-forming events we will be studying in this chapter all happen in the phosphate part of the molecule. You will see structural drawings of ATP, ADP, and AMP abbreviated in many different ways in this text and throughout the biochemical literature, depending on what is being illustrated. For example, the three structures below are all abbreviated depictions of ATP:
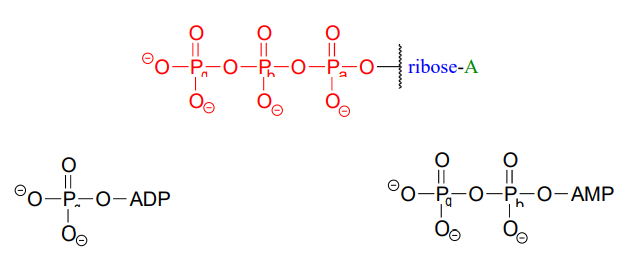
The following exercise will give you some practice in recognizing different abbreviations for ATP and other biological molecules that contain phosphate groups.
Exercise \(\PageIndex{1}\)
Below are a number of representations, labeled A-S, of molecules that contain phosphate groups. Different abbreviations are used. Arrange A-S into groups of drawings that depict the same species (for example, group together all of the abbreviations which depict ATP).
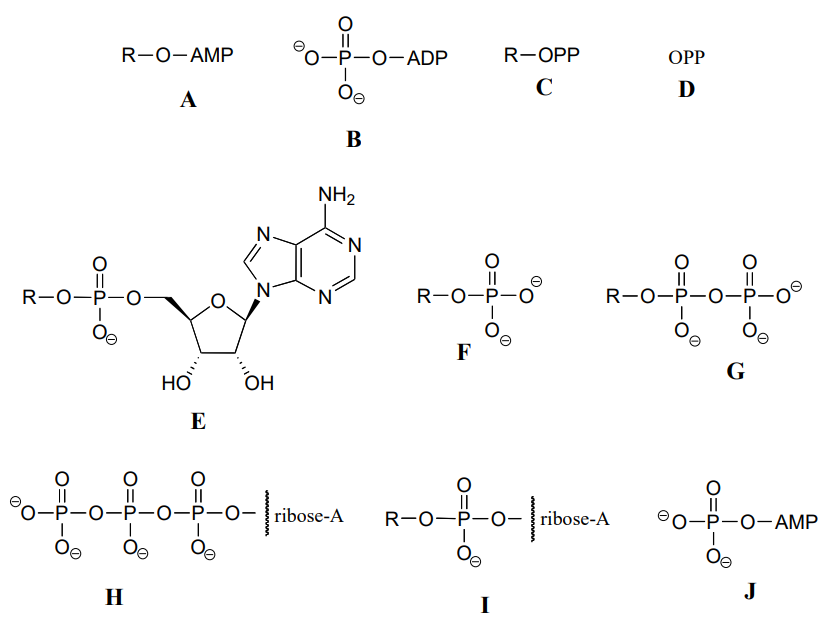
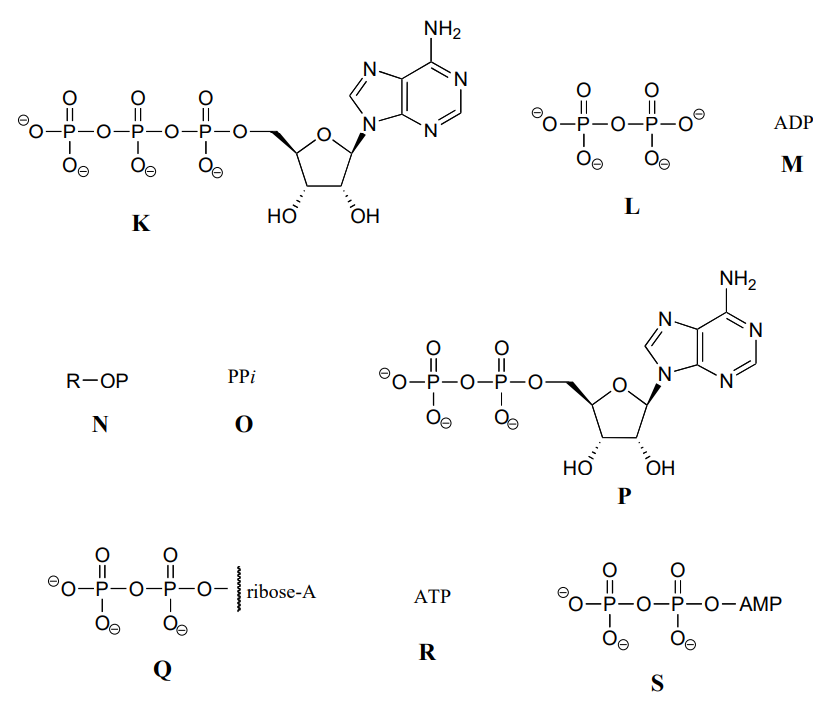
You are probably familiar with the physiological role of ATP from your biology classes - it is commonly called 'the energy currency of the cell'. What this means is that ATP stores energy we get from the oxidation of fuel molecules such as carbohydrates or fats. The energy in ATP is stored in the two high-energy phosphate anhydride linkages.

When one or both of these phosphate anhydride links are broken as a phosphate group is transferred to an acceptor, a substantial amount of energy is released. The negative charges on the phosphate groups are separated, eliminating some of electrostatic repulsion that existed in ATP. One way to picture this is as a coil springing open, releasing potential energy.
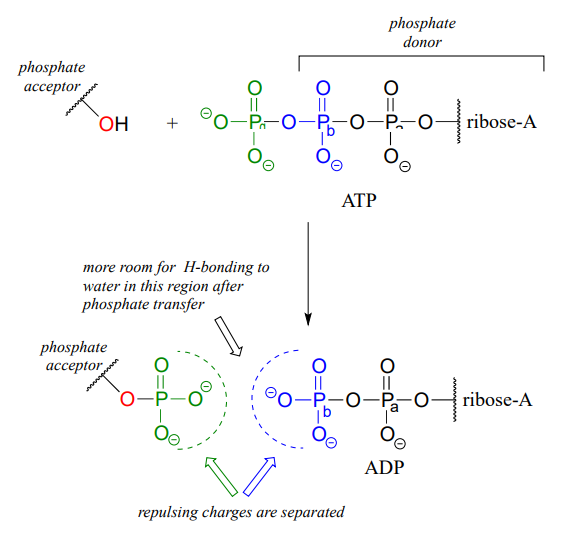
In addition, cleavage of a phosphate anhydride bond means that surrounding water molecules are able to form more stabilizing hydrogen-bonding interactions with the products than was possible with the starting materials, again making the reaction more 'downhill', or exergonic.
It is important to understand that while the phosphate anhydride bonds in ATP are thermodynamically unstable (they contain a great deal of chemical energy), they are at the same time kinetically stable: ATP-cleaving reactions are exothermic, but also have a high energy barrier, making them very slow unless catalyzed by an enzyme. In other words, the release of the energy contained in ATP is highly energetic but also subject to tight control by the interaction of highly evolved enzymes in our metabolic pathways.
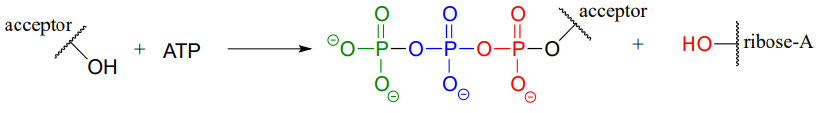
ATP is a versatile phosphate group donor: depending on the site of nucleophilic attack (at the \(\alpha \), \(\beta \), or \(\gamma \) phosphorus), different phosphate transfer outcomes are possible. Below are the three most common patterns seen in the central metabolic pathways. A 'squigly' line in each figure indicates the \(P-O\) bond being broken. We will study specific examples of each of these in the coming sections.
Attack at the \(\gamma \)-phosphate:
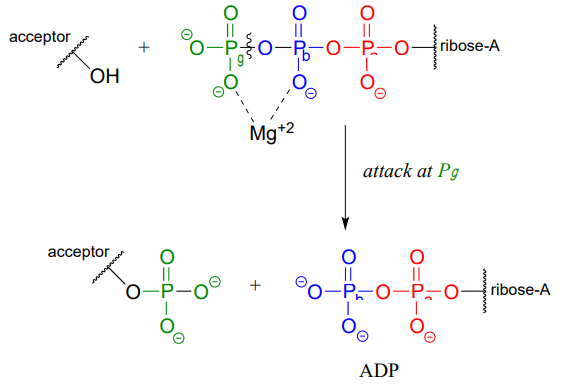
Attack at the \(\beta \)-phosphate:
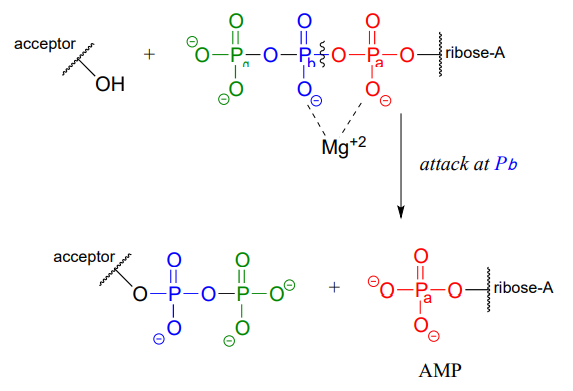
Attack at the \(\alpha \)-phosphate:
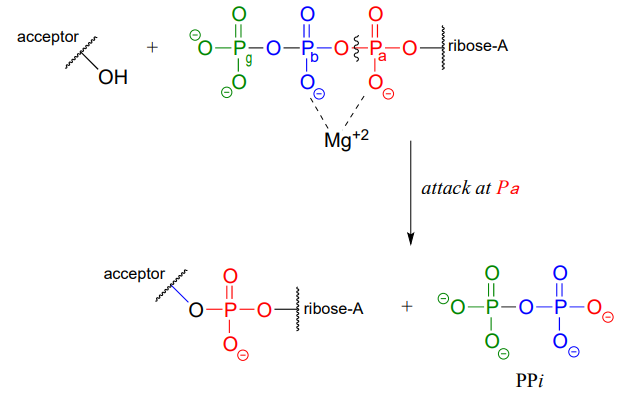
Note
The common thread running through all of the ATP-dependent reactions we will see in this section is the idea that the phosphate acceptor molecule is undergoing a thermodynamically 'uphill' transformation to become a more reactive species. The energy for this uphill transformation comes from breaking a high-energy phosphate anhydride bond in ATP. That is why ATP is often referred to as 'energy currency': the energy in its anhydride bonds is used to 'pay for' a thermodynamically uphill chemical step.
Exercise \(\PageIndex{2}\)
Propose a fourth hypothetical phosphate transfer reaction between ATP and the generic acceptor molecule in the figure above, in which inorganic phosphate (Pi) is a by-product.
Exercise \(\PageIndex{3}\)
Why is this hypothetical phosphate transfer reaction less energetically favorable compared to all of the possible ATP-cleaving reactions shown in the figure above?
Contributors
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


