3.2b. Electron Affinity
- Page ID
- 107493
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative ion. In other words, the neutral atom's likelihood of gaining an electron.
Introduction
Energy of an atom is defined when the atom loses or gains energy through chemical reactions that cause the loss or gain of electrons. A chemical reaction that releases energy is called an exothermic reaction and a chemical reaction that absorbs energy is called an endothermic reaction. Energy from an exothermic reaction is negative, thus energy is given a negative sign; whereas, energy from an endothermic reaction is positive and energy is given a positive sign. An example that demonstrates both processes is when a person drops a book. When he or she lifts a book, he or she gives potential energy to the book (energy absorbed). However, once the he or she drops the book, the potential energy converts itself to kinetic energy and comes in the form of sound once it hits the ground (energy released).
When an electron is added to a neutral atom (i.e., first electron affinity) energy is released; thus, the first electron affinities are negative. However, more energy is required to add an electron to a negative ion (i.e., second electron affinity) which overwhelms any the release of energy from the electron attachment process and hence, second electron affinities are positive.
- First Electron Affinity (negative energy because energy released):
\[ \ce{X (g) + e^- \rightarrow X^{-} (g)} \label{1}\]
- Second Electron Affinity (positive energy because energy needed is more than gained):
\[ \ce{X^- (g) + e^- \rightarrow X^{2-} (g)} \label{2}\]
First Electron Affinity
Ionization energies are always concerned with the formation of positive ions. Electron affinities are the negative ion equivalent, and their use is almost always confined to elements in groups 16 and 17 of the Periodic Table. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous -1 ions. It is the energy released (per mole of X) when this change happens. First electron affinities have negative values. For example, the first electron affinity of chlorine is -349 kJ mol-1. By convention, the negative sign shows a release of energy.
When an electron is added to a metal element, energy is needed to gain that electron (endothermic reaction). Metals have a less likely chance to gain electrons because it is easier to lose their valance electrons and form cations. It is easier to lose their valence electrons because metals' nuclei do not have a strong pull on their valence electrons. Thus, metals are known to have lower electron affinities.
Example \(\PageIndex{1}\): Group 1 Electron Affinities
This trend of lower electron affinities for metals is described by the Group 1 metals:
- Lithium (Li): 60 KJ mol-1
- Sodium (Na): 53 KJ mol-1
- Potassium (K): 48 KJ mol-1
- Rubidium (Rb): 47 KJ mol-1
- Cesium (Cs): 46 KJ mol-1
Notice that electron affinity decreases down the group.
When nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); thus, the electron affinity will be negative. Nonmetals have a greater electron affinity than metals because of their atomic structures: first, nonmetals have more valence electrons than metals do, thus it is easier for the nonmetals to gain electrons to fulfill a stable octet and secondly, the valence electron shell is closer to the nucleus, thus it is harder to remove an electron and it easier to attract electrons from other elements (especially metals). Thus, nonmetals have a higher electron affinity than metals, meaning they are more likely to gain electrons than atoms with a lower electron affinity.
Example \(\PageIndex{2}\): Group 17 Electron Affinities
For example, nonmetals like the elements in the halogens series in Group 17 have a higher electron affinity than the metals. This trend is described as below. Notice the negative sign for the electron affinity which shows that energy is released.
- Fluorine (F) -328 kJ mol-1
- Chlorine (Cl) -349 kJ mol-1
- Bromine (Br) -324 kJ mol-1
- Iodine (I) -295 kJ mol-1
Notice that electron affinity decreases down the group, but increases up with the period.
As the name suggests, electron affinity is the ability of an atom to accept an electron. Unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gas atom. The more negative the electron affinity value, the higher an atom's affinity for electrons.
Periodic Table showing Electron Affinity Trend
Nonmetals vs. Metals
To summarize the difference between the electron affinity of metals and nonmetals (Figure \(\PageIndex{1}\)):
- Metals: Metals like to lose valence electrons to form cations to have a fully stable octet. They absorb energy (endothermic) to lose electrons. The electron affinity of metals is lower than that of nonmetals.
- Nonmetals: Nonmetals like to gain electrons to form anions to have a fully stable octet. They release energy (exothermic) to gain electrons to form an anion; thus, electron affinity of nonmetals is higher than that of metals.
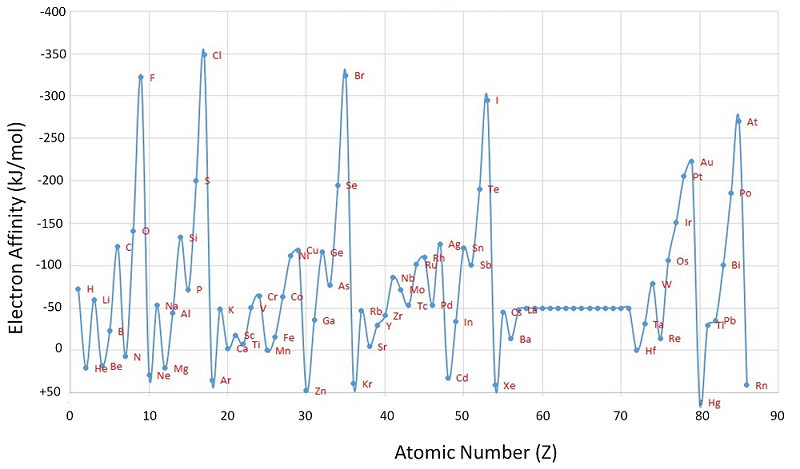
Figure \(\PageIndex{1}\): A Plot of Periodic Variation of Electron Affinity with Atomic Number for the First Six Rows of the Periodic Table. Notice that electron affinities can be both negative and positive. Image used with permission from Robert J. Lancashire (University of the West Indies).
Patterns in Electron Affinity
Electron affinity increases upward for the groups and from left to right across periods of a periodic table because the electrons added to energy levels become closer to the nucleus, thus a stronger attraction between the nucleus and its electrons. Remember that greater the distance, the less of an attraction; thus, less energy is released when an electron is added to the outside orbital. In addition, the more valence electrons an element has, the more likely it is to gain electrons to form a stable octet. The less valence electrons an atom has, the least likely it will gain electrons.
Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far from the nucleus, thus a decrease from its pull. However, one might think that since the number of valence electrons increase going down the group, the element should be more stable and have higher electron affinity. One fails to account for the shielding affect. As one goes down the period, the shielding effect increases, thus repulsion occurs between the electrons. This is why the attraction between the electron and the nucleus decreases as one goes down the group in the periodic table.
As you go down the group, first electron affinities become less (in the sense that less energy is evolved when the negative ions are formed). Fluorine breaks that pattern, and will have to be accounted for separately. The electron affinity is a measure of the attraction between the incoming electron and the nucleus - the stronger the attraction, the more energy is released. The factors which affect this attraction are exactly the same as those relating to ionization energies - nuclear charge, distance and screening. The increased nuclear charge as you go down the group is offset by extra screening electrons. Each outer electron in effect feels a pull of 7+ from the center of the atom, irrespective of which element you are talking about.
Example \(\PageIndex{3}\): Fluorine vs. Chlorine
A fluorine atom has an electronic structure of 1s22s22px22py22pz1. It has 9 protons in the nucleus.The incoming electron enters the 2-level, and is screened from the nucleus by the two 1s2 electrons. It therefore feels a net attraction from the nucleus of 7+ (9 protons less the 2 screening electrons).
In contrast, chlorine has the electronic structure 1s22s22p63s23px23py23pz1 with 17 protons in the nucleus. But again the incoming electron feels a net attraction from the nucleus of 7+ (17 protons less the 10 screening electrons in the first and second levels). There is also a small amount of screening by the 2s electrons in fluorine and by the 3s electrons in chlorine. This will be approximately the same in both these cases and so does not affect the argument in any way (apart from complicating it!).
The over-riding factor is therefore the increased distance that the incoming electron finds itself from the nucleus as you go down the group. The greater the distance, the less the attraction and so the less energy is released as electron affinity.
Comparing fluorine and chlorine is not ideal, because fluorine breaks the trend in the group. However, comparing chlorine and bromine, say, makes things seem more difficult because of the more complicated electronic structures involved. What we have said so far is perfectly true and applies to the fluorine-chlorine case as much as to anything else in the group, but there's another factor which operates as well which we haven't considered yet - and that over-rides the effect of distance in the case of fluorine.
Why is Fluorine an Anomaly?
The incoming electron is going to be closer to the nucleus in fluorine than in any other of these elements, so you would expect a high value of electron affinity. However, because fluorine is such a small atom, you are putting the new electron into a region of space already crowded with electrons and there is a significant amount of repulsion. This repulsion lessens the attraction the incoming electron feels and so lessens the electron affinity. A similar reversal of the expected trend happens between oxygen and sulfur in Group 16. The first electron affinity of oxygen (-142 kJ mol-1) is smaller than that of sulfur (-200 kJ mol-1) for exactly the same reason that fluorine's is smaller than chlorine's.
Comparing Group 16 and Group 17 values
As you might have noticed, the first electron affinity of oxygen (\(-142\; kJ\; mol^{-1}\)) is less than that of fluorine (\(-328\; kJ\; mol^{-1}\)). Similarly sulfur's (\(-200\; kJ\; mol^{-1}\)) is less than chlorine's (\(-349\; kJ\; mol^{-1}\)). Why? It's simply that the Group 16 element has 1 less proton in the nucleus than its next door neighbor in Group 17. The amount of screening is the same in both. That means that the net pull from the nucleus is less in Group 16 than in Group 17, and so the electron affinities are less.
The reactivity of the elements in group 17 falls as you go down the group - fluorine is the most reactive and iodine the least. Often in their reactions these elements form their negative ions. The first impression that is sometimes given that the fall in reactivity is because the incoming electron is held less strongly as you go down the group and so the negative ion is less likely to form. That explanation looks reasonable until you include fluorine!
An overall reaction will be made up of lots of different steps all involving energy changes, and you cannot safely try to explain a trend in terms of just one of those steps. Fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in other steps in its reactions more than makes up for the lower amount of energy released as electron affinity.
Second Electron Affinity
You are only ever likely to meet this with respect to the group 16 elements oxygen and sulfur which both form -2 ions. The second electron affinity is the energy required to add an electron to each ion in 1 mole of gaseous 1- ions to produce 1 mole of gaseous 2- ions. This is more easily seen in symbol terms.
\[ X^- (g) + e^- \rightarrow X^{-2} (g) \label{3}\]
It is the energy needed to carry out this change per mole of \(X^-\).
Why is energy needed to do this? You are forcing an electron into an already negative ion. It's not going to go in willingly!
\[ O_{g} + e^- \rightarrow O^- (g) \;\;\; \text{1st EA = -142 kJ mol}^{-1} \label{4}\]
\[ O^-_{g} + e^- \rightarrow O^{2-} (g) \;\;\; \text{2nd EA = +844 kJ mol}^{-1} \label{5}\]
The positive sign shows that you have to put in energy to perform this change. The second electron affinity of oxygen is particularly high because the electron is being forced into a small, very electron-dense space.
Practice Problems
- When an electron is added to a nonmetal atom, is energy released or absorbed?
- Why do nonmetal atoms have a greater electron affinity than metal atoms?
- Why are atoms with a low electron affinity more likely to lose electrons than gain electrons?
- As you move down a group of the periodic table, does electron affinity increase or decrease, if so, why?
- Why do nonmetals want to gain electrons?
- Why do metals have a low electron affinity?
Answers
- Energy is released when a electron is added to a nonmetal.
- Nonmetals have a greater electron affinity than metals because their atomic structure allows them to gain electrons rather than lose them.
- Atoms with a low electron affinity want to give up their valence electrons because they are further from the nucleus; as a result, they do not have a strong pull on the valence electrons.
- As you move down a group on the periodic table, electron affinity decreases. First, the electrons are placed in energy levels further away from the nucleus, which results in electrons not having a strong attraction to the nucleus; secondly, the atom does not want gain electrons because there is minimal charge on the outer energy levels from the nucleus; and lastly, the shielding effect increases, causing repulsion between the electrons, thus they move further from each other and the nucleus itself.
- Nonmetals want to gain electrons because they have more valence electrons than metals, so it is easier for them to gain electrons than lose the valance electrons to fulfill a stable octet. In addition, nonmetals' valance electrons are closer to the nucleus, thus allowing more attraction between the two.
- Metals have a low electron affinity (a less likely chance to gain electrons) because they want to give up their valence electrons rather than gain electrons, which require more energy than necessary. In addition, they do not have a strong pull on the valance electrons because they are far away from the nucleus, thus they have less energy for an attraction.
References
- Petrucci, Harwood, Herring, Madura. General Chemistry Principles & Modern Applications. Prentice Hall. New Jersey, 2007.
- Tro, Nivaldo J. (2008). Chemistry: A Molecular Approach (2nd Edn.). New Jersey: 2008
- Myers, R. Thomas. "The periodicity of electron affinity." J. Chem. Educ. 1990, 67, 307.
- Wheeler, John C. " Electron Affinities of the Alkaline Earth Metals and the Sign Convention for Electron Affinity." J. Chem. Educ. 1997 74 123.
Contributors
- Harjeet Bassi (UCD), Nilpa Shah (UCD), Shelley Chu (UCD)
Jim Clark (Chemguide.co.uk)

