19.5: Retrosynthesis with Enolates
- Page ID
- 375463
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)By now, you’ve seen that enolates react with a variety of different functional groups:
1. aldehydes and ketones
2. esters, anhydrides, and acid chlorides
3. alkyl halides
4. \(α\),\(β\)-unsaturated carbonyls
The most difficult part of this section of the course is designing a synthesis. The strategy you should take is retrosynthesis, where you take a molecule and break it apart into smaller portions by making key bond disconnections. Recognizing that a particular functional groups can be made by a specific reaction will be important.
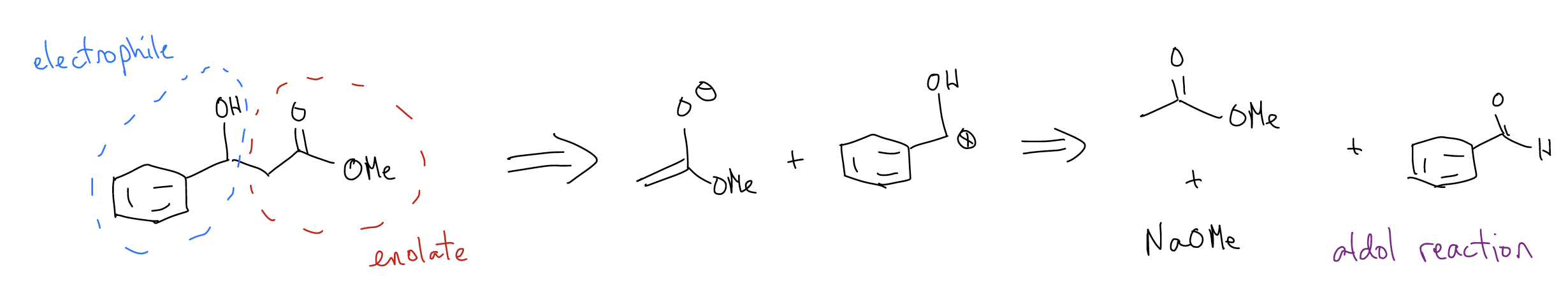
In this part of the course, the nucleophile is an enolate, so when you are making C-C bonds, one of those carbons will be capable of forming an enolate. You should simple circle this portion of the molecule so that you know that it’s what forms the enolate. The other carbon will be one of the four choices above. By looking at the particular C-C bond, you should be able to identify which electrophile you need. All that is left is to break the C-C bond into these components and draw an arrow with a set of reagents. Let’s do an example:
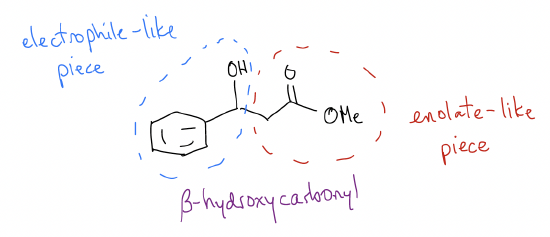
If we try to find an easy way to make this molecule, we should first try to identify where the enolate-like carbon is (it should be \(α\) to the carbonyl). There is only one choice, obviously, because there is only one carbonyl. This means that he other half is the electrophile. So, we must stitch together this molecule via C-C bond formation. Now we know that the enolate would look like this:
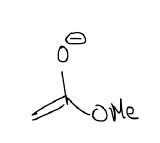
which can be made by deprotonating this ester with an appropriate base:

The only question we have left is – what is the electrophile? We know that our end product is an alcohol and the choices we have are: 1) aldehyde/ketone, 2) ester, 3) alkyl halide, or 4) \(α\),\(β\)-unsaturated carbonyl. Only one of these will work – the aldehyde!
This combination would be an aldol reaction between an ester and an aldehyde to product a \(β\)-hydroxyester. You can think of the electrophile portion of the molecule having reactivity similar to the structure below, which is equivalent to a carbonyl:
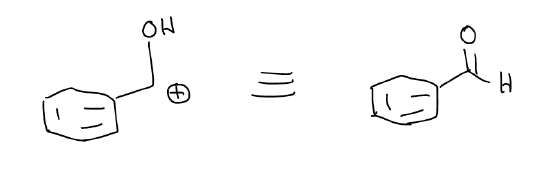
Let’s see if we can identify the enolate and electrophile in each case below:
1.
2.
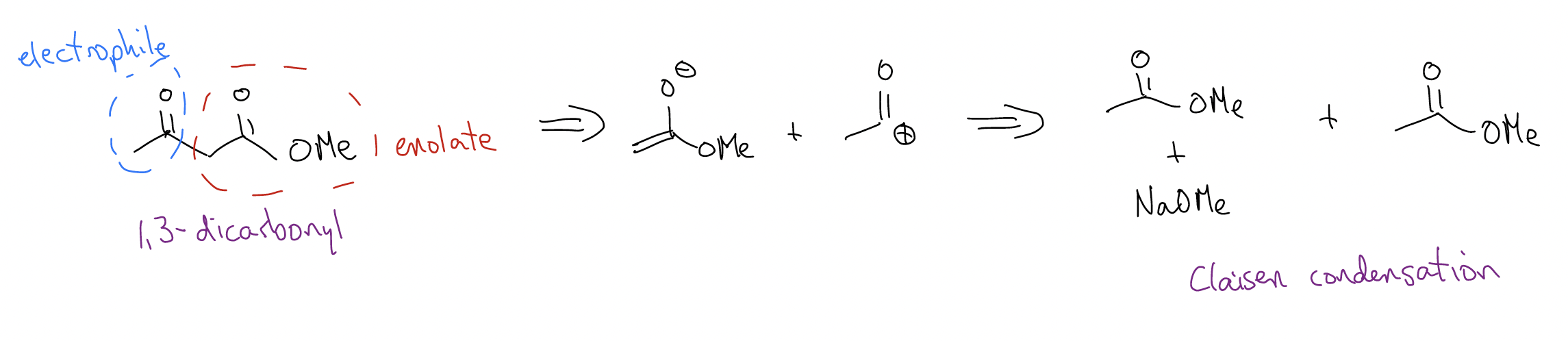
3.
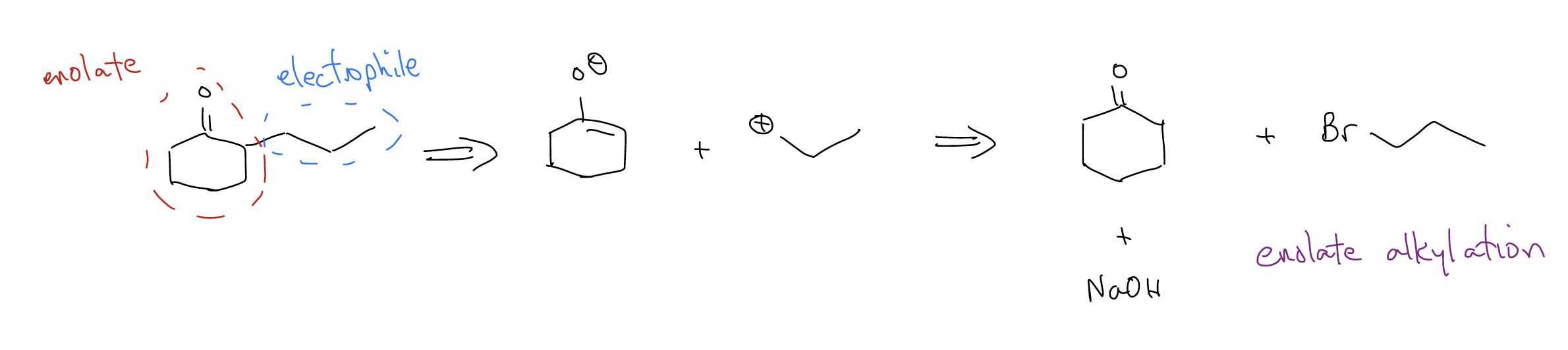
4.
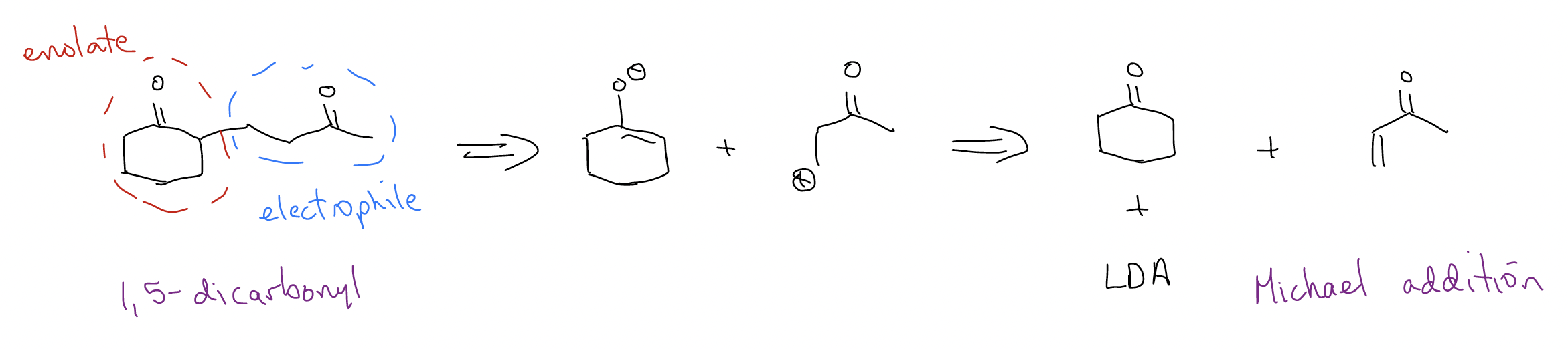
So, we’ve now developed a strategy for identifying important functional groups like \(β\)-hydroxycarbonyl (think, aldol!), \(β\)-ketoester or 1,3-dicarbonyl (think, Claisen condensation!), or 1,5-dicarbonyl (think, Michael addition). There is one other pesky functional group which gives us trouble and that’s the \(α\),\(β\)-unsaturated carbonyl – how do we form that? It’s a two-step process involving E1cb and aldol. Here’s an example:
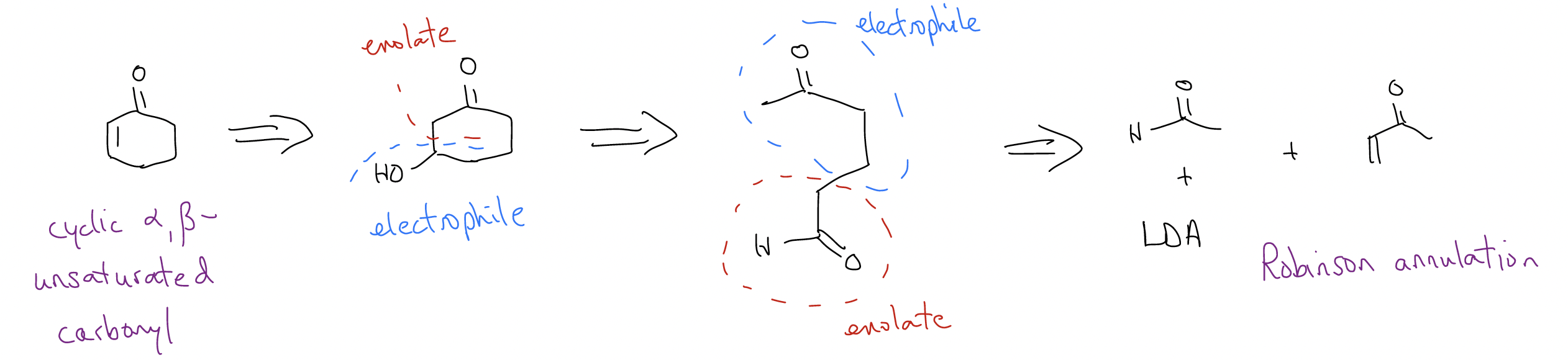
First, identify the double bond as one in conjugation with a carbonyl – this is important otherwise the rest of this doesn’t work. Alkenes are formed by elimination and the specific type of elimination operative here is an E1cb, or dehydration. This E1cb is possible because enolate formation is quick due to resonance stabilization from oxygen. This helps to push out OH–, so our regiochemistry should be an OH-group at the \(β\)-position. This should look familiar – isn’t a \(β\)-hydroxycarbonyl formed via an aldol reaction? If we do the same sort of C-C bond disconnection, we get ring opening to provide a 1,5-carbonyl. This should also look familiar – isn’t a 1,5-dicarbonyl formed via a Michael reaction? If we do a bond disconnection accordingly, we should get an aldehyde and an \(α\),\(β\)-unsaturated ketone. Without even realizing it, you’ve just completed a Robinson annulation! The key is to start by identifying the functional groups that are easy to form and break bonds until you arrive at something new that suggests another key bond disconnection.

