19.3: Reactions of Enolates with Alkyl Halides
- Page ID
- 375461
Reactions of enolates with alkyl halides
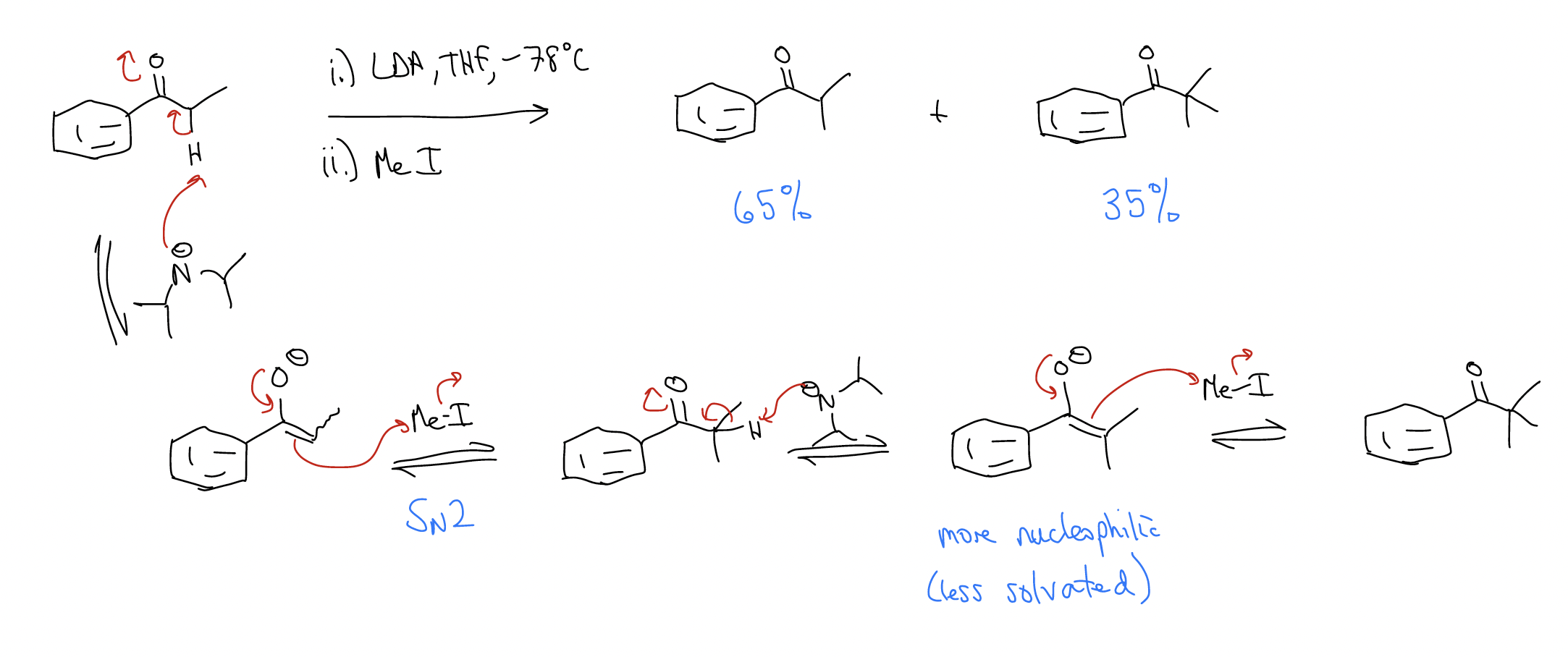
Alkylation of enolates – can occur when an enolate reacts via an SN2 mechanism with a low-lying \(σ^{*}\)C-X, but it is very difficult to stop at monoalkylation.
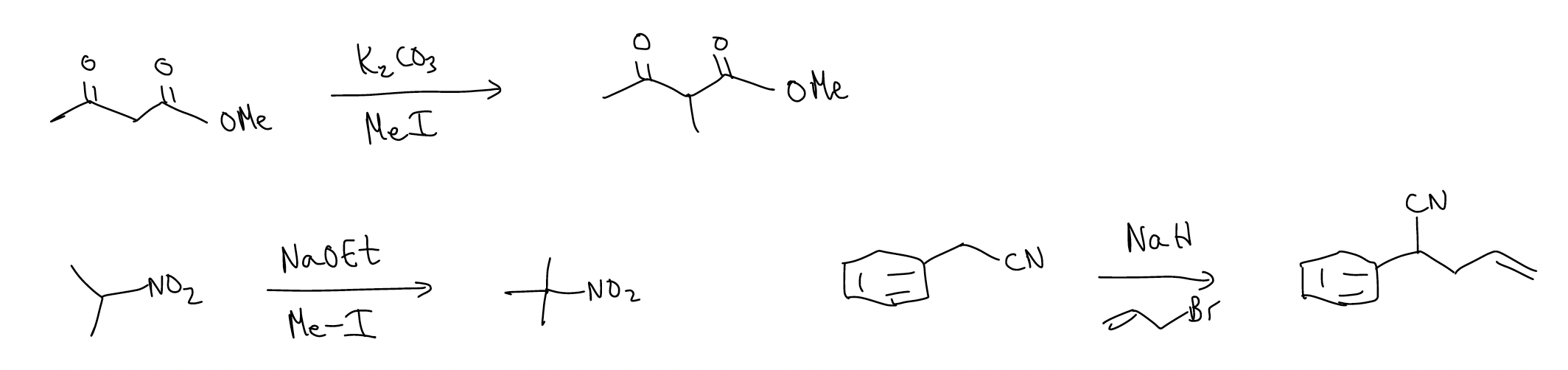
Other examples:
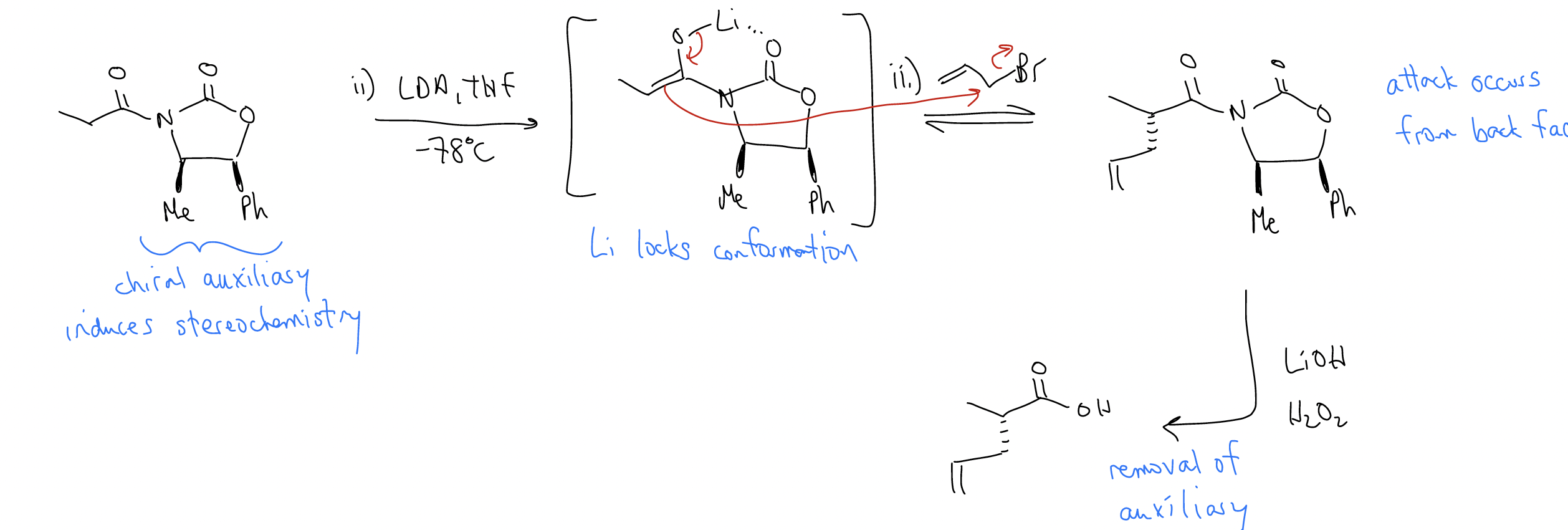
Reactions involving chiral enolates give us an opportunity to create chirality since any transition state that forms is diastereomeric. Consider the following reaction:
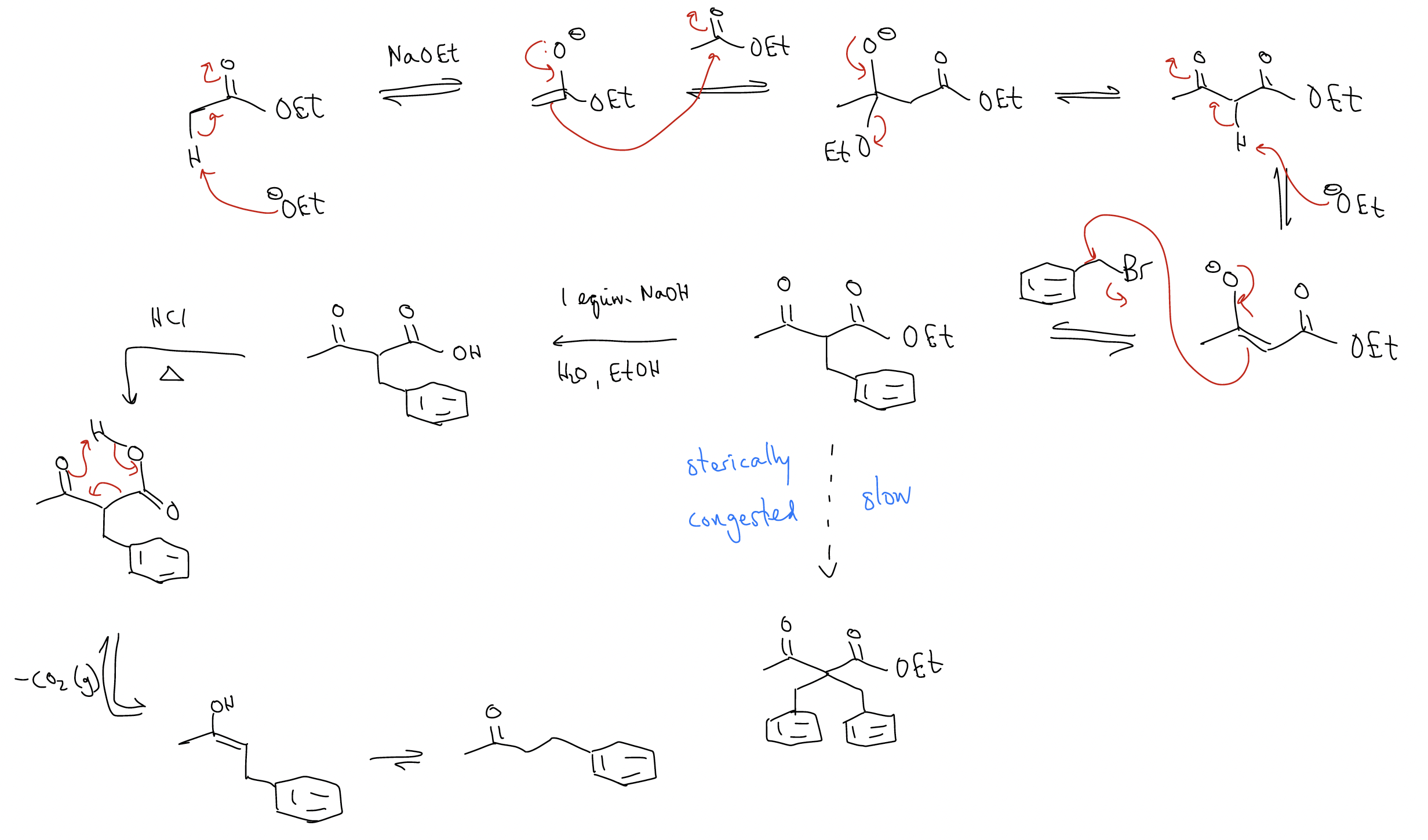
Let’s come back to this issue of double alkylation. Say we wanted to create the following complex ester by double alkylation of a simple ester. The problem is that a double alkylation product is formed.
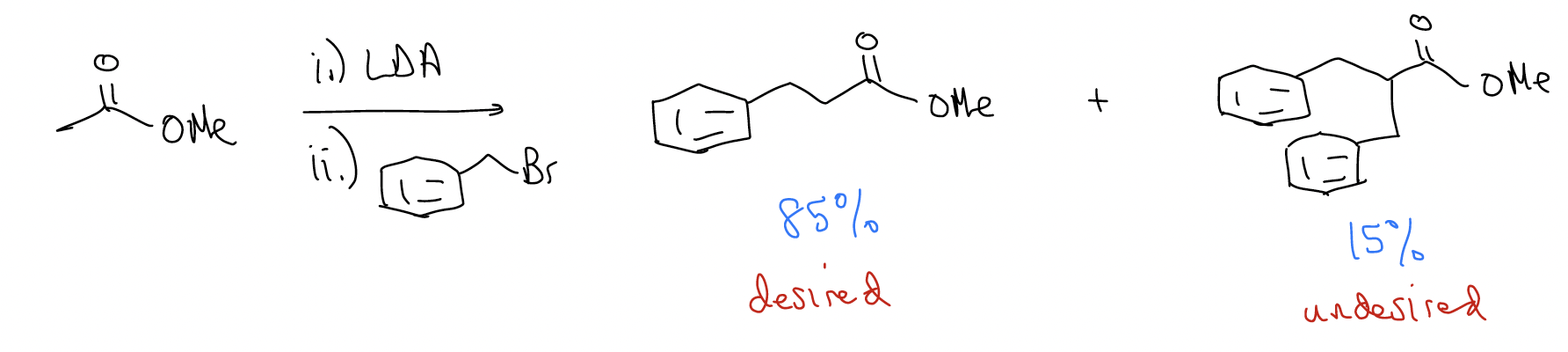
How do we avoid this? We perform a malonic ester synthesis:
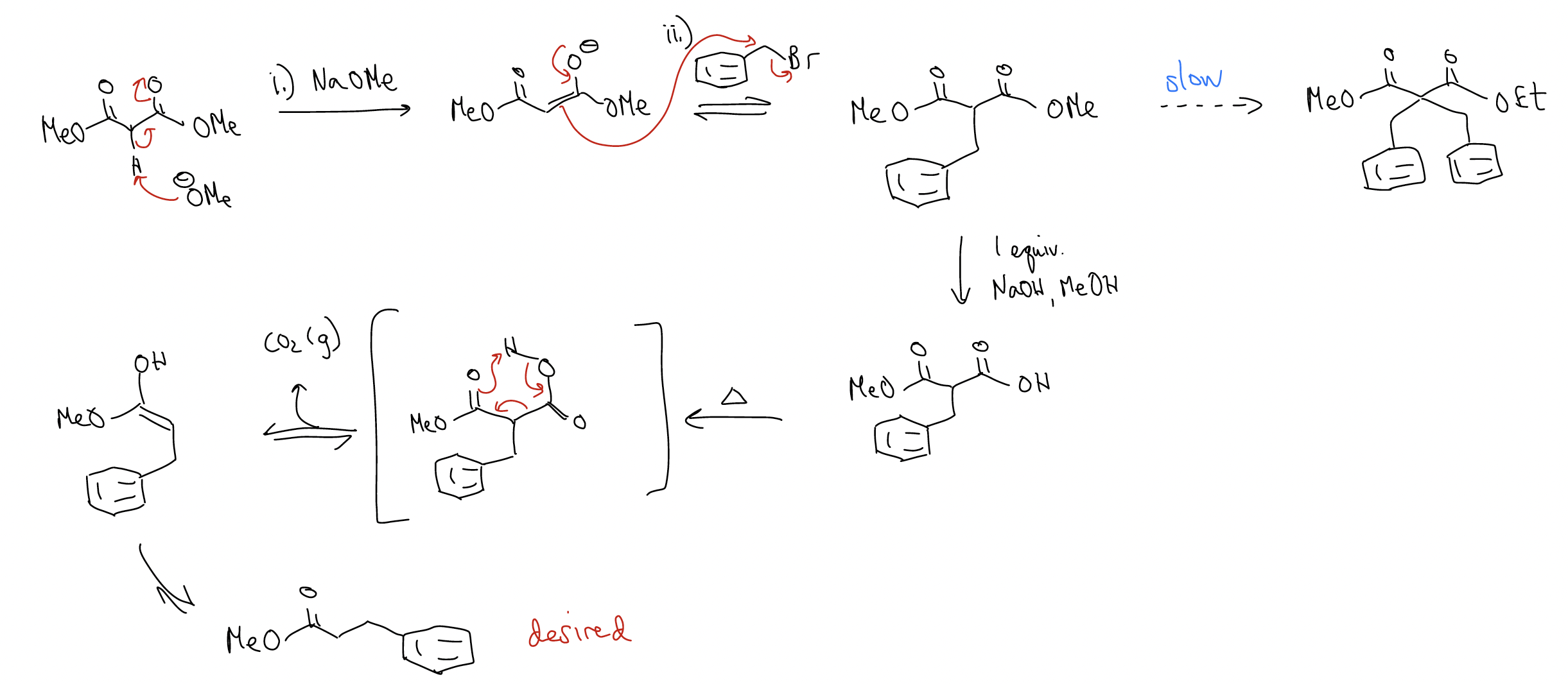
The malonic ester synthesis is a good way of making \(α\)-substituted esters and can be performed successively:
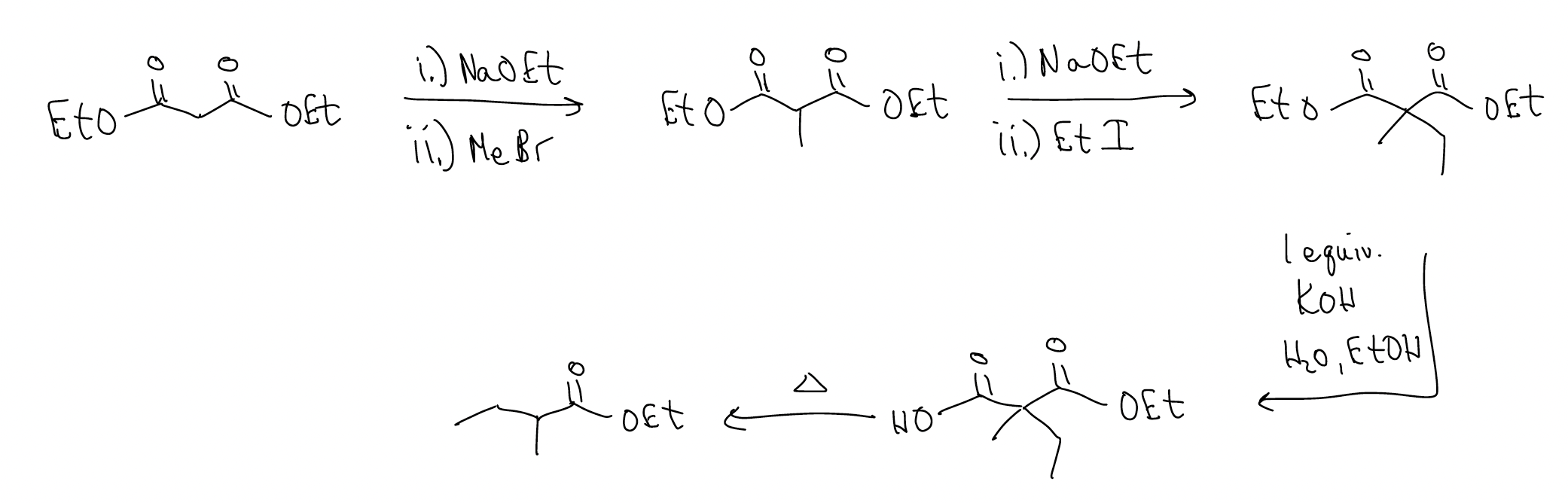
What about using a similar strategy for making differentially substituted ketones? We can accomplish this by using the acetoacetic ester synthesis, the first step of which is a Claisen condensation.