19.1: Reactions of Enolates with Aldehydes and Ketones
- Page ID
- 375459
Reactions of enolates with aldehydes/ketones
Aldol reaction – the formation of a \(β\)-hydroxycarbonyl upon reacting an aldehyde with an enolate anion. For example, treating propanal with a base (OH–) gives an aldol product by the following mechanism:
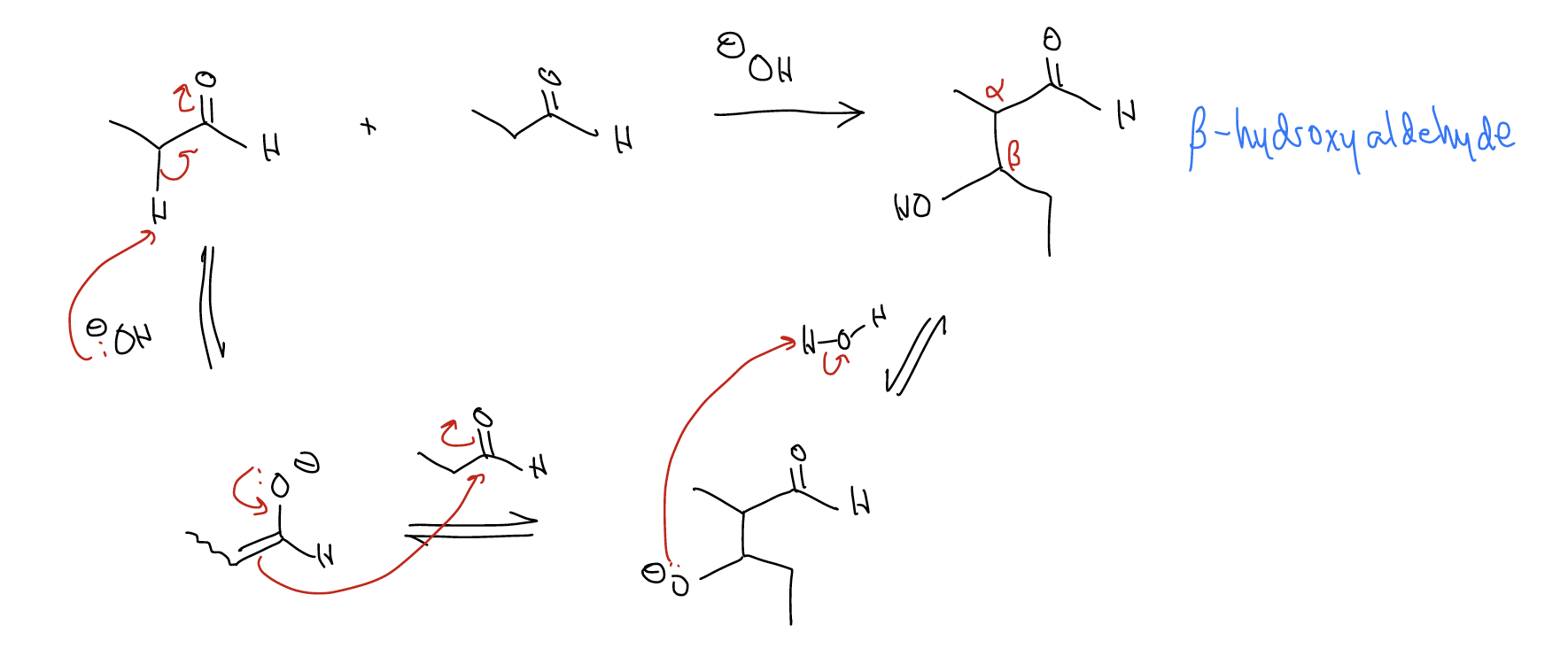
Think of the “\(β\)-hydroxyaldehyde” functional group as derived retrosynthetically from an aldol reaction. And remember that the enolate can be anything!
Mixed aldol condensation – an aldol reaction where the two carbonyls are not the same. Here, you must have the ability to funnel into only one product. Thus, you must limit the number of enolates you generate. We can accomplish this by using an aryl aldehyde that cannot form an enolate, according to the mechanism below:
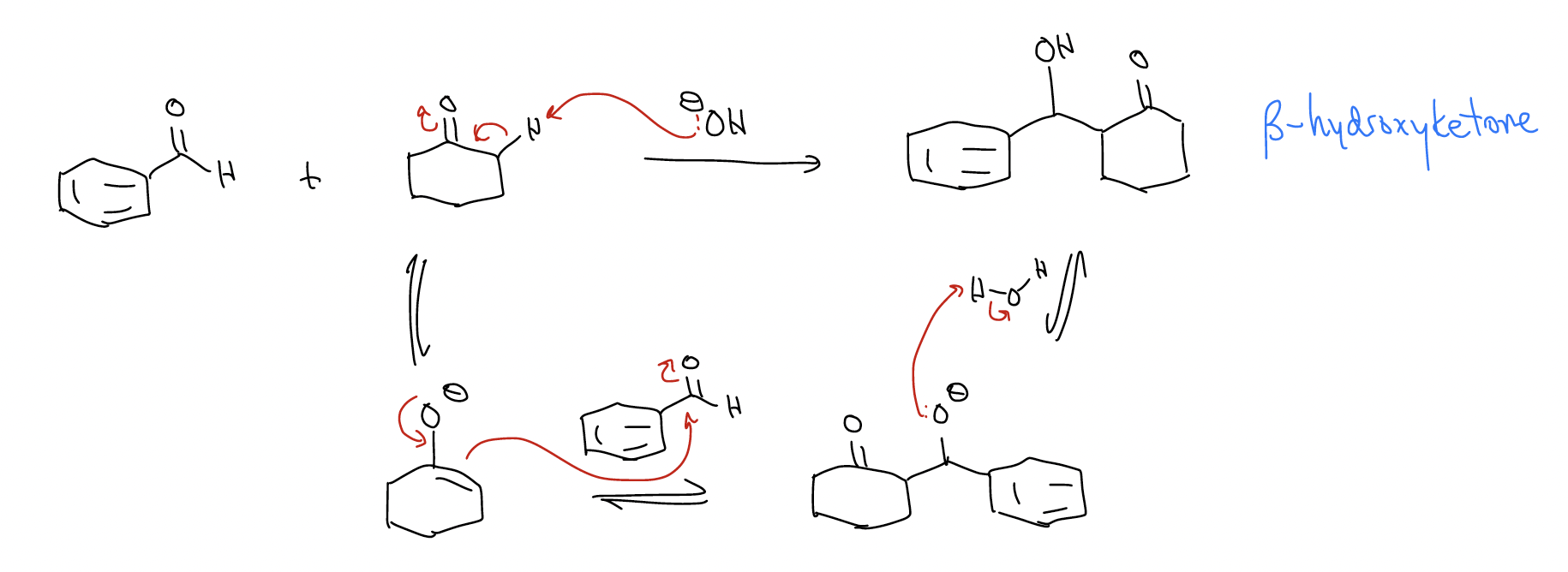
This mixed aldol reaction works because: 1) only one enolate can form, and 2) the aldehyde is more reactive (lower \(π^{*}\)) than the ketone. The reaction can also occur a second time if two equivalents of aldehyde are present.
The \(β\)-hydroxycarbonyl functional group is very unstable in most cases, however, and will eliminate water to give an aldol condensation product. When using ketones and aromatic aldehydes as substrates, this is known as the Claisen-Schmidt reaction, the product of which is a chalcone.
Claisen-Schmidt reaction – aldol condensation between a ketone and an aryl aldehyde requiring more forcing conditions (excess base and heat).
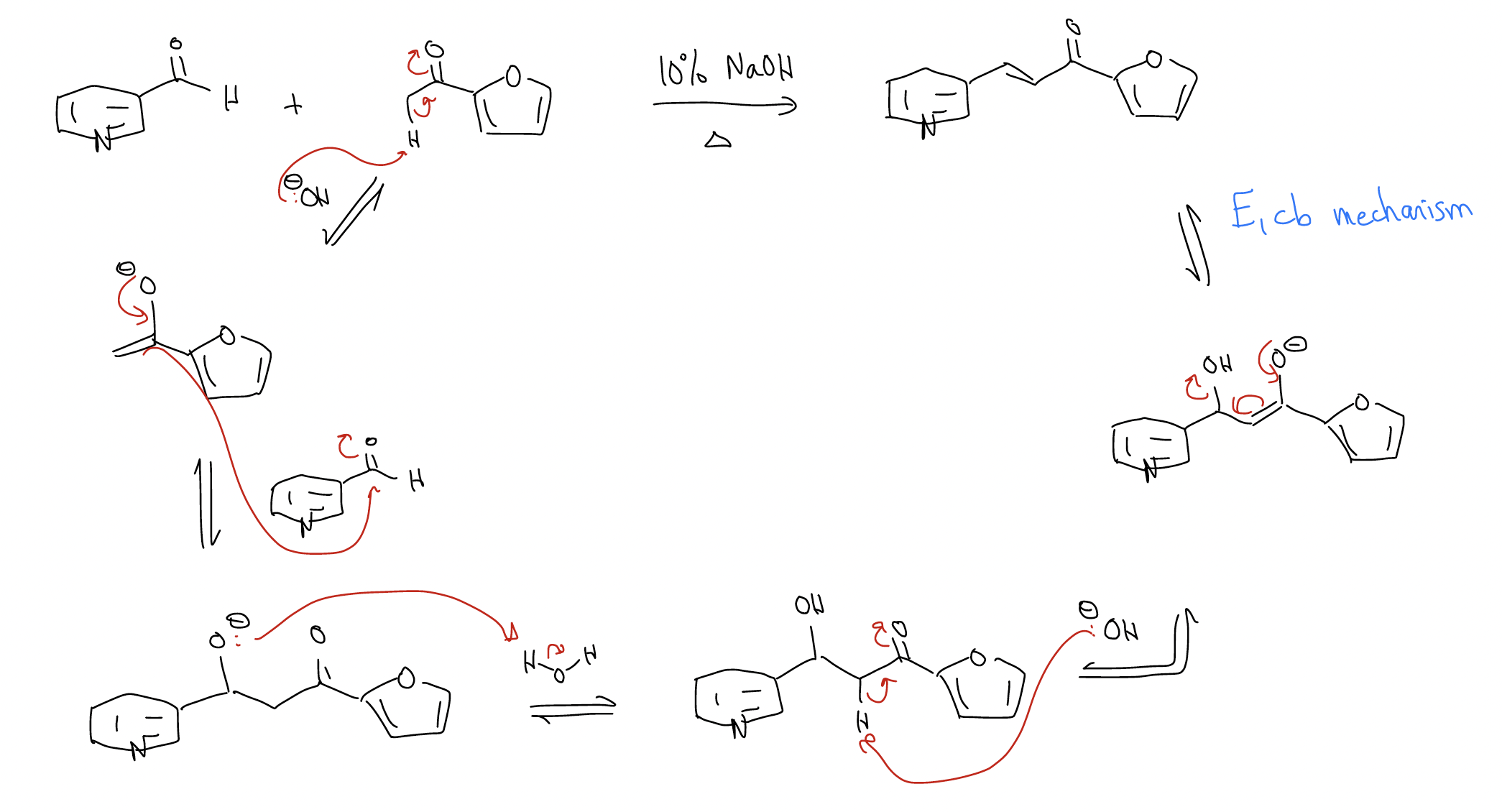
This reaction is under thermodynamic control. The \(α\),\(β\)-unsaturated carbonyl is an important structure retrosynthetically. The last step of this mechanism is known as an E1cb mechanism, where “cb” stands for conjugate base and the rate-limiting step is the elimination.
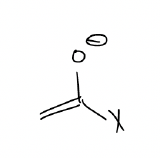
Mukaiyama aldol – an aldol reaction that involves an enolate-like intermediate known as the silyl enol ether. The advantage of using this reaction is that there is no risk of “scrambling.” Consider the following using LDA as the base:
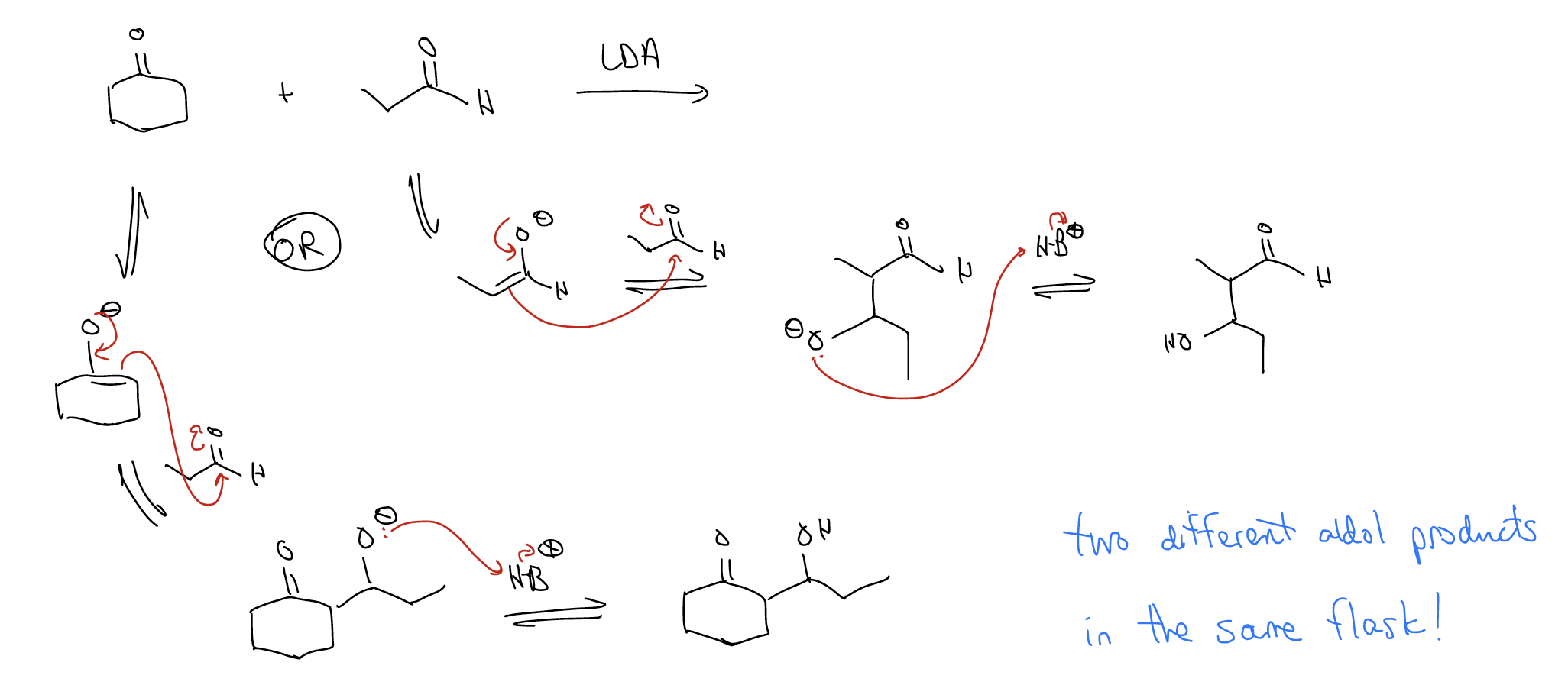
We can avoid the formation of two different aldol products by using a silyl enol ether:
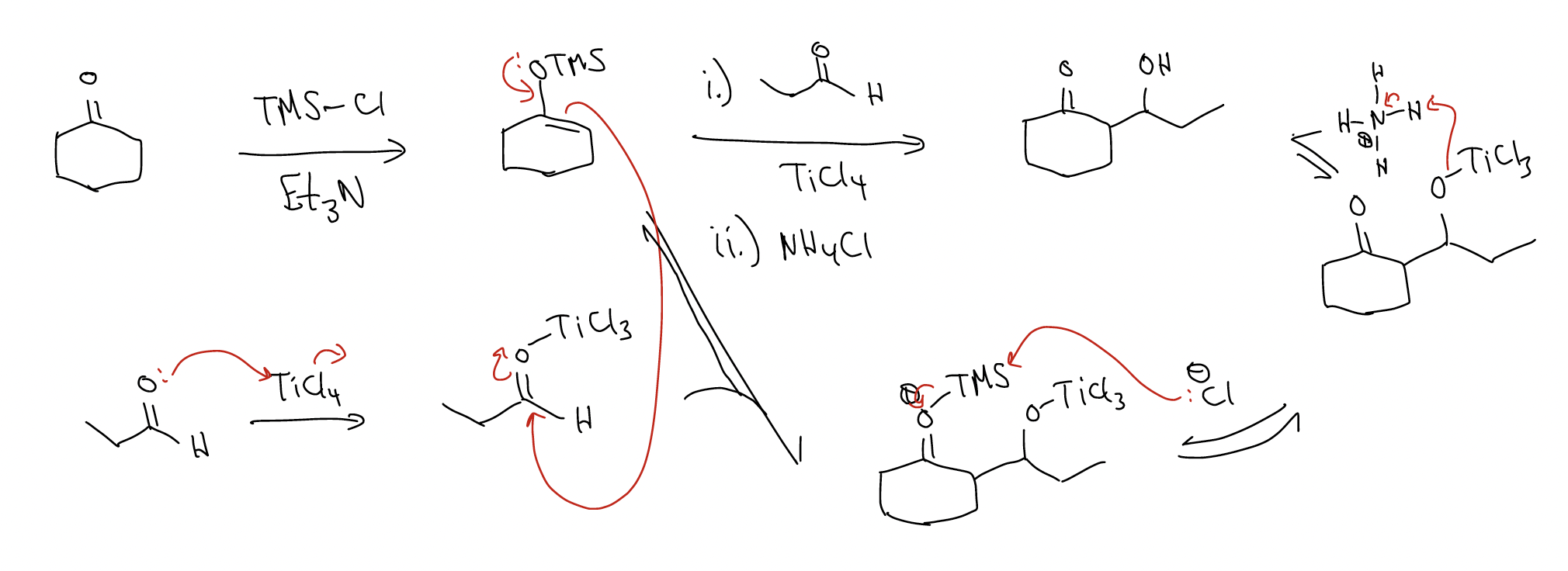
Other Lewis acids for this reaction include: BF3, ZnCl2, MgCl2

