16.5: Nucleophilic Addition Under Neutral Conditions
- Page ID
- 375446
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)C. Neutral conditions
1. Imine/enamine formation
Similar in mechanism to acetal formation is imine and enamine formation, except that these reactions normally occur under neutral conditions. Like acetal formation, H2O must be a leaving group. While this is not possible under basic conditions, strongly acidic conditions will just protonate the amine, leaving the reaction dead!
In imine formation, nucleophilic attack on the carbonyl results in a hemiaminal intermediate (carbinolamine).
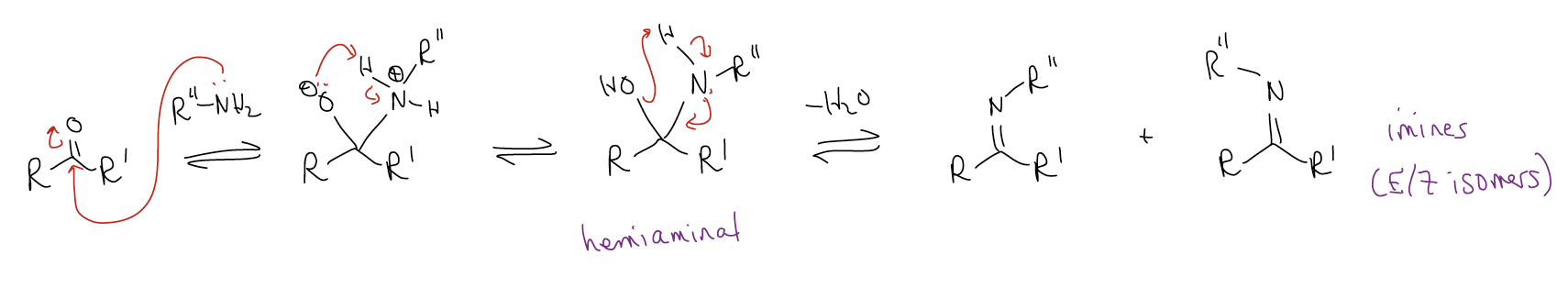
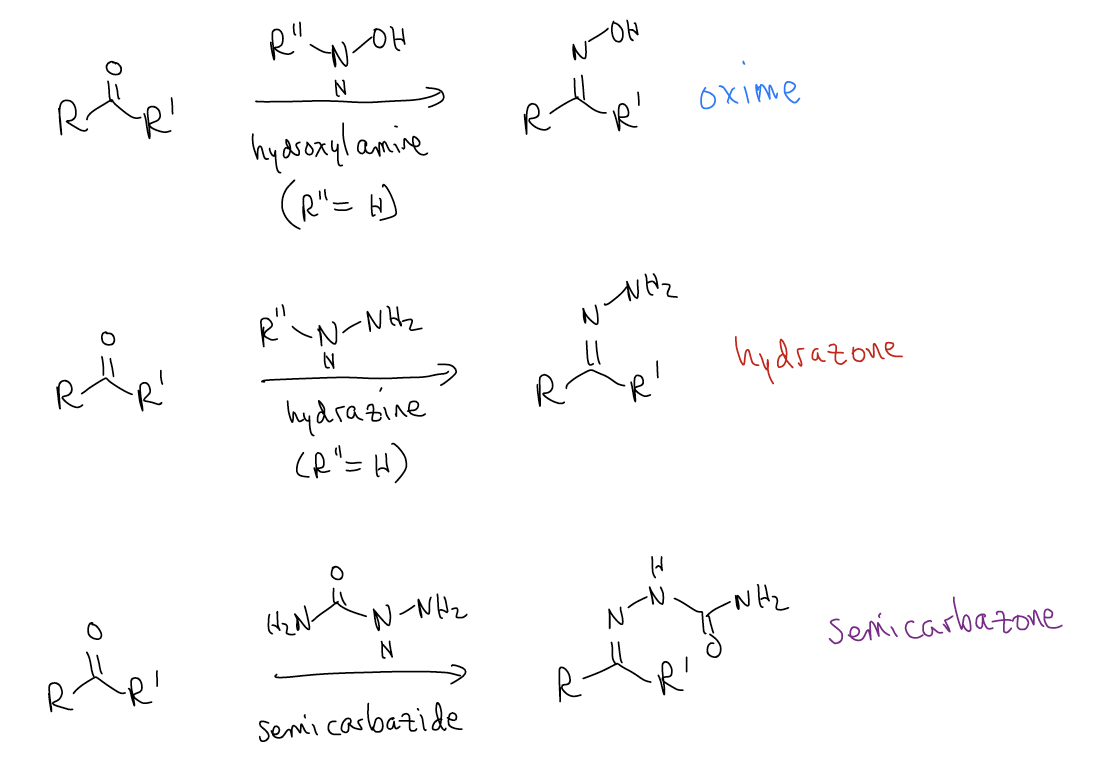
This reaction is usually run at pH 4-6. Acetic acid is normally added as a catalyst. Several different types of amine can be used, whose products have special names. In each of these cases, the amine is a primary amine, and H2O is removed. Thus, we call these reactions condensation reactions, but the mechanism is still addition to the \(π^{*}\)C-O.
When a secondary amine is used, the resulting iminium ion deprotonates to form an enamine. The most common secondary amines are pyrrolidine, piperidine, and morpholine, but dialkylamines of all types work. In order for enamine formation to proceed, the ketone must have \(α\)-protons.
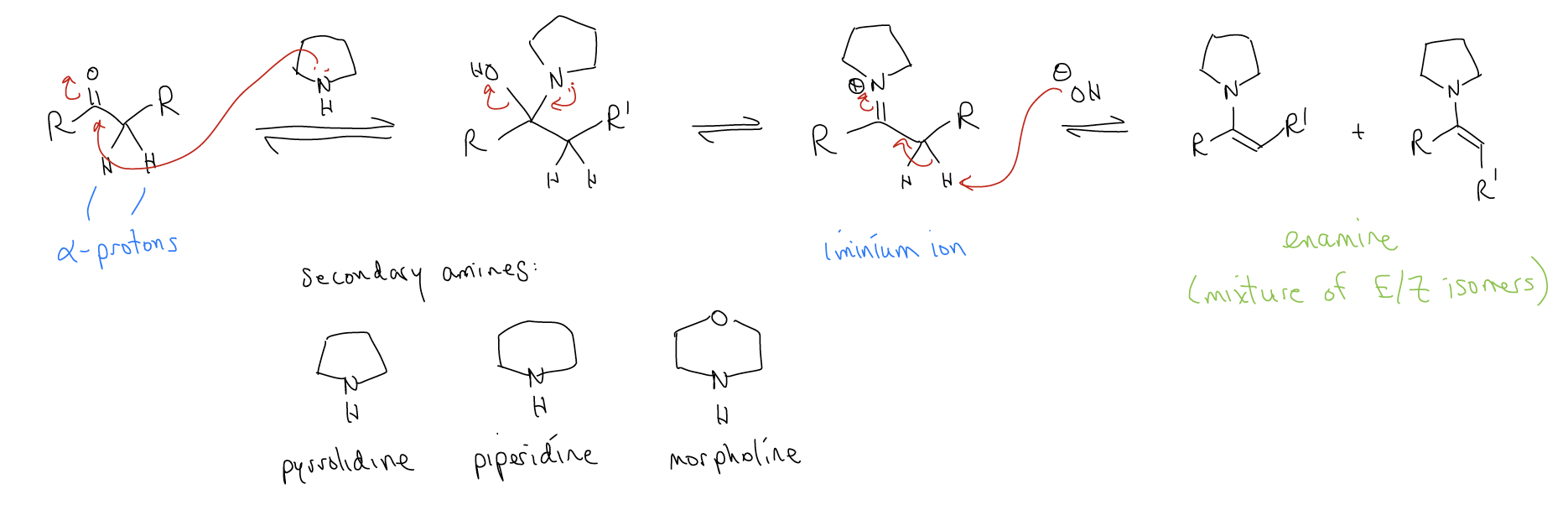
We will come back to describe the synthetic uses of enamines later, but first a brief detour on the use of imines in synthesis. Recall our discussion of the synthesis of amines during SN1/SN2 chemistry. We said that because of hyperconjugation, it is very difficult to stop the reaction of a primary amine with an alkyl halide at monoalkylation – the reaction keeps going because the product is more reactive than the starting material.
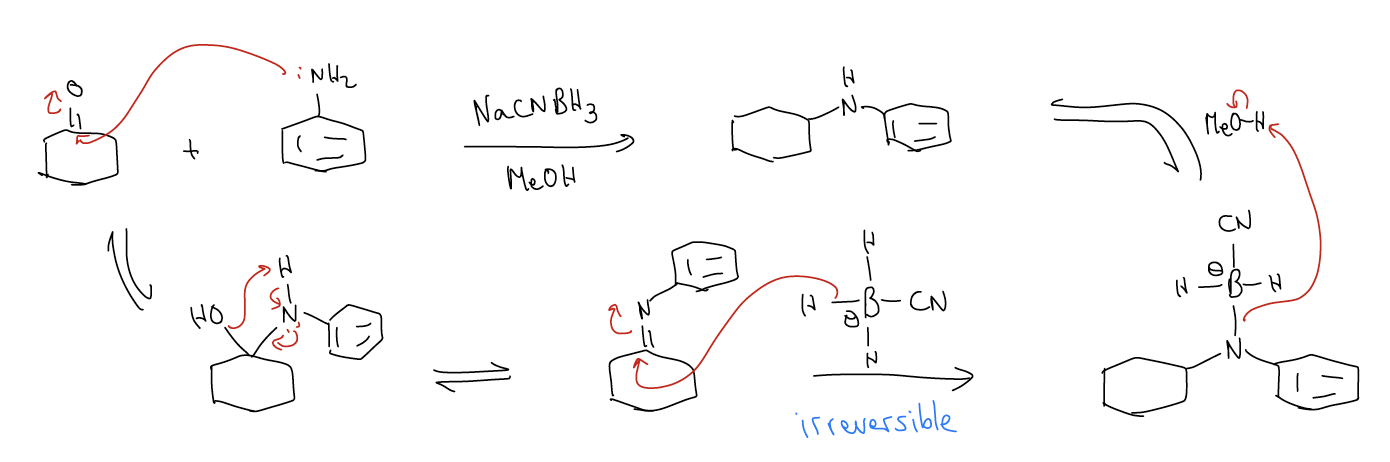
One way to circumvent this problem is to use the imine functional group as a handle. A good way to make secondary amines is by a process called reductive amination of a ketone/aldehyde. We know that a ketone/aldehyde will react with a primary amine to give an imine, so what if we could reduce the imine with a reducing agent like NaBH4 to yield the secondary amine? It turns out that you could do this, but it would take you two steps – formation of the imine, followed by reduction with NaBH4. We need two steps because NaBH4 will reduce the ketone/aldehyde to the alcohol.
But as the name of this reaction implies, you can do this all in one reaction flask by decreasing the reducing agents reactivity. There are two reagents that do this: sodium cyanoborohydride (NaCNBH3) and sodium triacetoxyborohydride (NaBH(OAc)3). These reagents will not reduce the ketone/aldehyde, but are reactive enough to reduce the imine formed in situ. The net result: a secondary amine!
2. Wittig reaction
One final (and important) reaction of carbonyls is the Wittig olefination reaction. In this reaction, a ketone/aldehyde reacts with a phosphorous ylide to generate an alkene.
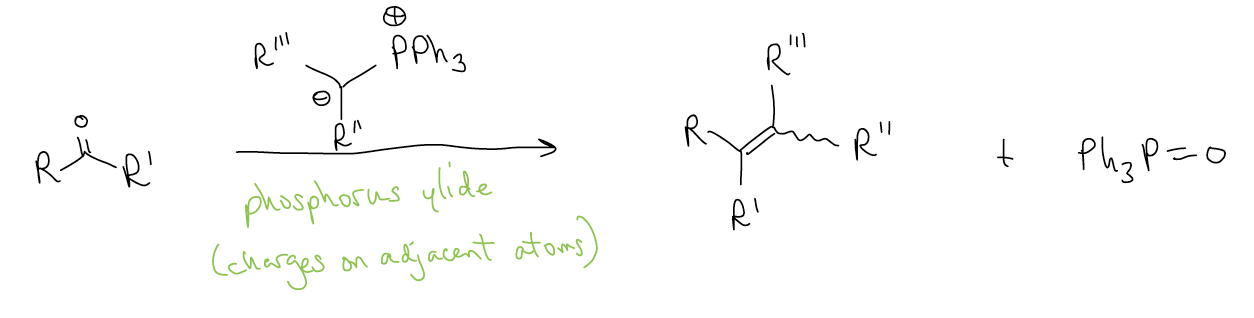
Formation of the ylide occurs by SN2, then deprotonation:
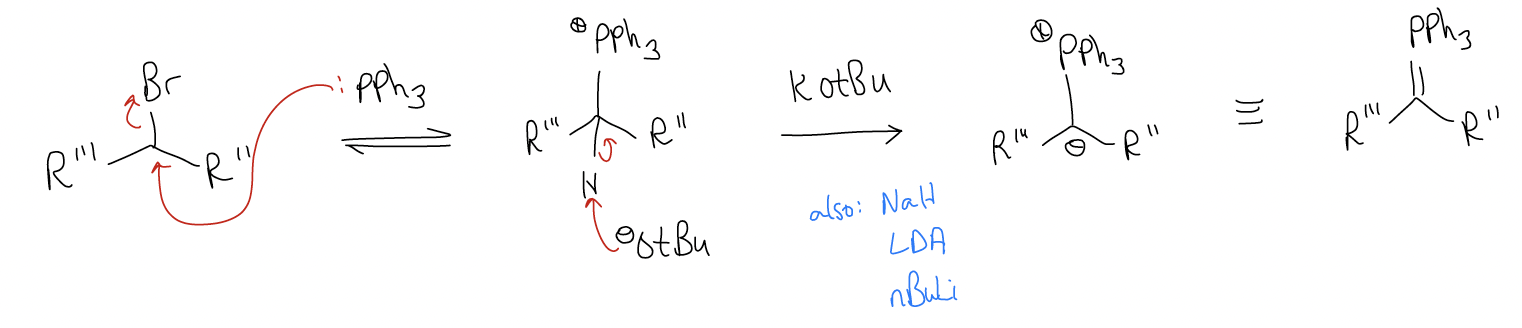
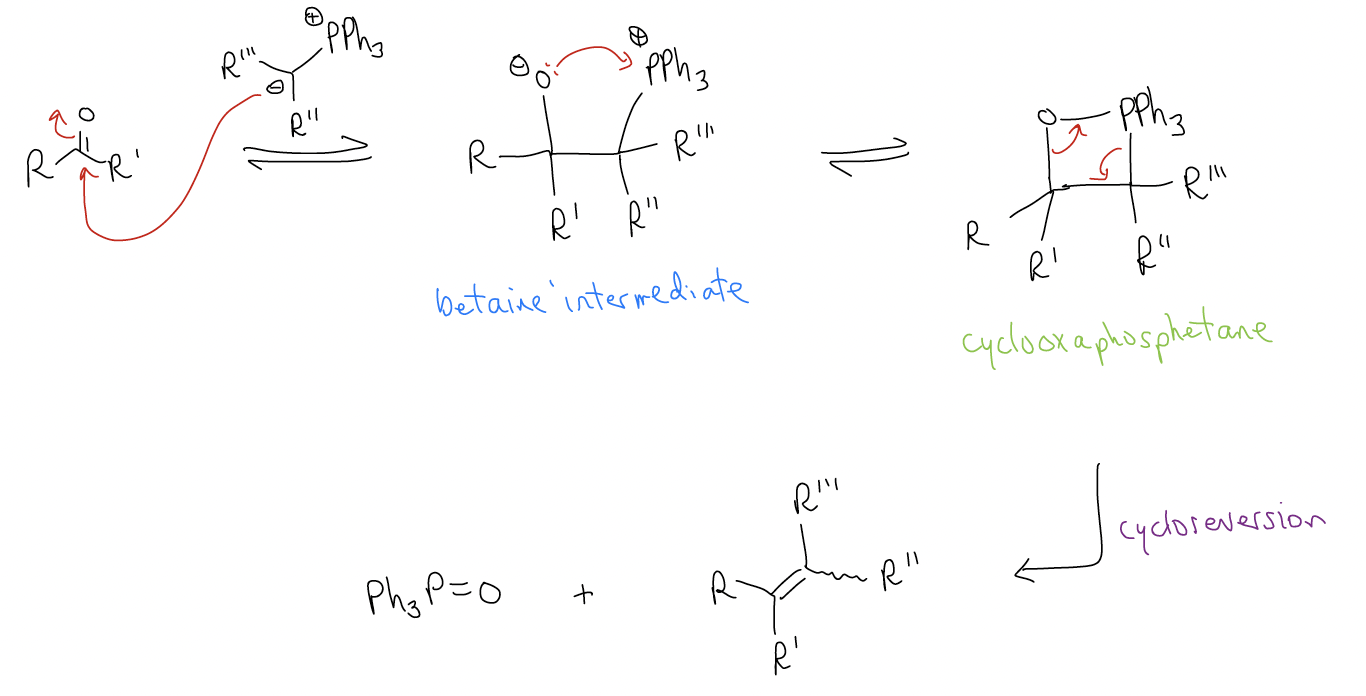
Treatment of the ylide (normally in situ) with a ketone/aldehyde results in nucleophilic addition to form a betaine intermediate, which cyclizes to give a cyclooxaphosphetane, then undergoes cycloreversion to yield the alkene and triphenylphosphine oxide.
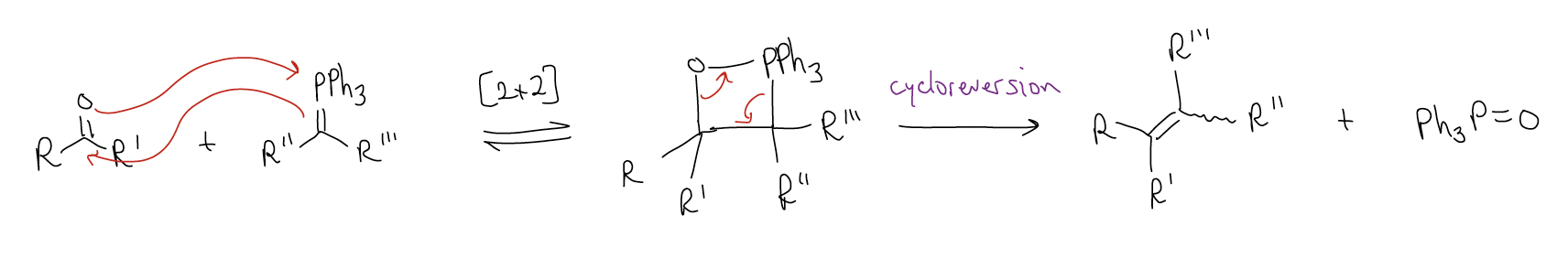
There is debate whether the betaine actually exists, so an alternative mechanism has been proposed, which involves the direct [2+2] cycloaddition to give the cyclooxaphosphetane.
As for considerations of stereochemistry, a general rule is that stabilized ylides give E-alkenes while unstabilized ylides give Z-alkenes. Stabilized ylides normally have an electron-withdrawing group on them, making the attack on the carbonyl reversible and favoring an anti orientation of the alkyl groups (thermodynamic control).

