9.9: A Very Brief Introduction to Redox Reactions
- Page ID
- 367628
- Associate the term redox with electron transfer.
- Recognize that a redox reaction is occurring when an atom is present in its elemental state on one side of a reaction and as part of a compound on the other side of the reaction.
"Redox" is short for "oxidation and reduction." Redox reactions are a large family of chemical reactions that involve transfer of electrons from one species to another. To simplify this complex topic for now, we will only look at redox reactions when an atom appears in its elemental form on one side of a chemical reaction and as part of a compound on the other side.
In all oxidation-reduction reactions an exchange of electrons occurs - one substance loses electrons while something else gains them. That is the key to understanding redox reactions.
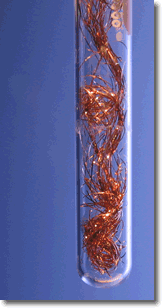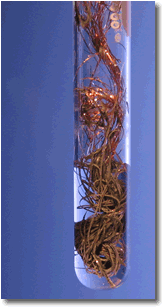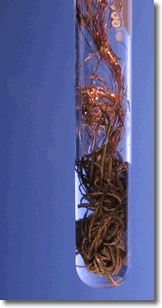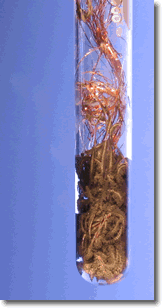
A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. Within minutes the wire begins to look fuzzy or furry, as small silver crystals begin to form on the wire. Meanwhile, the originally clear silver nitrate solution begins to take on a pale bluish tint. Furthermore, if the crystals are shaken off of the wire we see that the wire partially disintegrated.
The overall equation for our demonstration describes the events:
\[\ce{Cu(s) + 2AgNO3(aq) → Cu(NO3)2 (aq) + 2 Ag(s)}\]
In a simplified representation of this reaction, we can look at what changes are occuring with the copper and silver atoms:
\[\ce{Cu(s) + 2Ag^{+}(aq) → Cu^{2+}(aq) + 2Ag(s)}\]
The copper is changing from its neutral, elemental form to one of its ionic forms. For the silver, it is changing from its ionic form to its elemental form. These transformations necessitate an exchange of electrons.
Oxidation of Copper Metal to Make Copper Ions
Copper began as a neutral atom with no charge, but changed into an ion with a charge of +2. An atom becomes a positive ion by losing electrons:
\[\ce{Cu(s) → Cu^{2+}(aq) + 2e^{-}} \label{oxeq} \]
Notice that copper began as a solid but is converted into aqueous ions - this is why the copper wire disintegrates. We say that copper was oxidized because is has lost electrons (i.e., electrons appear on the product side of the Equation \(\ref{oxeq}\)).
Reduction of Silver Ions to Make Silver Metal
Silver was converted from an ion with a charge of +1, Ag+, to a neutral atom, Ag. The only way an ion can undergo this change is to gain an electron:
\[\ce{Ag^{+}(aq) + e^{-} → Ag(s)} \label{redeq}\]
Notice that solid silver is formed - this is what causes the fuzzy appearance to begin appearing on the wire - solid silver crystals. Silver has gained electrons - it has been reduced (i.e., electrons appear on the reactant side of Equation \(\ref{redeq}\)).
The electrons that silver gained had to come from somewhere - they came from copper. Conversely, a substance such as copper can only lose electrons if there is something else that will take them up, the silver ions. One cannot occur without the other. This exchange of electrons is what defines an oxidation - reduction reaction.
Oxidation is defined as the loss of electrons and reduction is defined as the gain of electrons.

