5.2: The Equation for a Harmonic-Oscillator Model of a Diatomic Molecule Contains the Reduced Mass of the Molecule
- Page ID
- 210813
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)For studying the energetics of molecular vibration we take the simplest example, a diatomic heteronuclear molecule \(\ce{AB}\). Let the respective masses of atoms \(\ce{A}\) and \(\ce{B}\) be \(m_A\) and \(m_B\). For diatomic molecules, we define the reduced mass \(\mu_{AB}\) by:
\[ \mu_{AB}=\dfrac{m_A\, m_B}{m_A+m_B} \label{5.2.1}\]
Reduced mass is the representation of a two-body system as a single-body one. When the motion (displacement, vibrational, rotational) of two bodies are only under mutual interactions, the inertial mass of the moving body with respect to the body at rest can be simplified to a reduced mass.
Reduced Mass
Viewing the multi-body system as a single particle allows the separation of the motion: vibration and rotation, of the particle from the displacement of the center of mass. This approach greatly simplifies many calculations and problems.
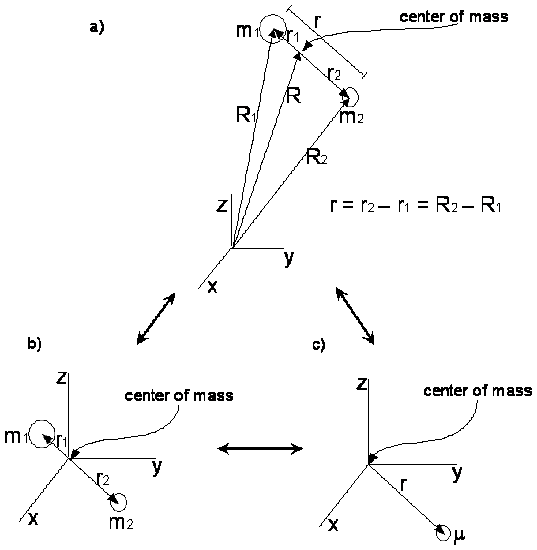
This concept is readily used in the general motion of diatomics, i.e. simple harmonic oscillator (vibrational displacement between two bodies, following Hooke's Law), the rigid rotor approximation (the moment of inertia about the center of mass of a two-body system), spectroscopy, and many other applications.
Determine the reduced mass of the two body system of a proton and electron with \(m_{proton} = 1.6727 \times 10^{-27}\, kg\) and \(m_{electron} = 9.110 \times 10^{-31}\, kg\)).
- Answer
-
\[\begin{align*} \mu_{pe} &= \dfrac{(1.6727 \times 10^{-27})(9.110 \times 10^{-31})}{1.6727 \times 10^{-27} + 9.110 \times 10^{-31}} \\[4pt] &= 9.105 \times 10^{-31} kg \end{align*}\]
The Quantum Harmonic Oscillator
The classical Harmonic Oscillator approximation is a simple yet powerful representation of the energetics of an oscillating spring system. Central to this model is the formulation of the quadratic potential energy
\[V(x) \approx \dfrac {1}{2} kx^2 \label{potential}\]
One problem with this classical formulation is that it is not general. We cannot use it, for example, to describe vibrations of diatomic molecules, where quantum effects are important. This require the formulation for Schrödinger Equation using Equation \ref{potential}.
\[ \hat{H} | \psi \rangle = \left[ \dfrac {-\hbar}{2\mu} \dfrac {d^2 }{d x^2} + \dfrac {1}{2}kx^2 \right ] | \psi \rangle = E|\psi \rangle\]
Solving this quantum harmonic oscillator is appreciably harder than the simplier Schrödinger Equation for the particle in the box mode and is outside the scope of this text. However, as with most quantum modules (and in contrast to the classical harmonic oscillator), the energies are quantized in terms of the quantum number \(v\)
\[ \begin{align} E_v &= \hbar \left(\sqrt {\dfrac {k}{\mu}} \right) \left(v + \dfrac {1}{2} \right) \\[4pt] &= h \nu \left(v+\dfrac {1}{2} \right) \end{align} \]
with the natural vibrational frequency of the system given as
\[\nu = \dfrac{1}{2 \pi}\sqrt {\dfrac {k}{\mu}}\]
and the mass, \(\mu\), is the reduced mass of the system (Equation \ref{5.2.1}).
Be careful to distinguish \(\nu\), the symbol for the natural frequency (as a Greek nu) from \(v\) the quantum harmonic oscillator quantum number (Latin v).
Contributors
- Eugene Lee
- stackExchange

