4.1: Chemical Bonding
- Page ID
- 217260
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Define ionic and molecular (covalent) compounds
- Predict the type of compound formed from elements based on their location within the periodic table
- Determine formulas for simple ionic compounds
- Identify polyatomic ions
Atomic Bonding
Chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood theory that would not only explain why atoms bind together to form molecules, but would also predict the three-dimensional structures of the resulting compounds as well as the energies and other properties of the bonds themselves. Unfortunately, no one theory exists that accomplishes these goals in a satisfactory way for all of the many categories of compounds that are known. Moreover, it seems likely that if such a theory does ever come into being, it will be far from simple.
When we are faced with a scientific problem of this complexity, experience has shown that it is often more useful to concentrate instead on developing models. A scientific model is something like a theory in that it should be able to explain observed phenomena and to make useful predictions. But whereas a theory can be discredited by a single contradictory case, a model can be useful even if it does not encompass all instances of the phenomena it attempts to explain.
Given the extraordinary variety of ways in which atoms combine into aggregates, it should come as no surprise that a number of useful bonding models have been developed. Most of them apply only to certain classes of compounds, or attempt to explain only a restricted range of phenomena. In this section we will provide brief descriptions of some of the bonding models.
Video \(\PageIndex{1}\): A brief overview of the bonds we will discuss below.
Ionic Bonding and Ionic Compounds
When an element composed of atoms that readily lose electrons (a metal) reacts with an element composed of atoms that readily gain electrons (a nonmetal), a transfer of electrons usually occurs, producing ions. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, Na+, and each chlorine atom in a sample of chlorine gas (group 17) accepts one electron to form a chloride anion, Cl−, the resulting compound, NaCl, is composed of sodium ions and chloride ions in the ratio of one Na+ ion for each Cl− ion. The solid NaCl, therefore consists of positive and negative ions arranged in a three - dimensional structure, called a crystal lattice. Each ion is attracted to neighboring ions of opposite charge, and is repelled by ions of like charge; this combination of attractions and repulsions, acting in all directions, causes the ion to be tightly fixed in its own location in the crystal lattice.
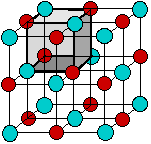
Figure \(\PageIndex{1}\): A model of a crystal lattice structure
Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form CaCl2, which is composed of Ca2+ and Cl− ions in the ratio of one Ca2+ ion to two Cl− ions.
A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the compound is usually ionic. This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course. However, it is not always true (for example, aluminum chloride, AlCl3, is not ionic).
Video \(\PageIndex{2}\): What is an ionic bond?
Ionic compounds can often be recognized by their properties. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. For example, sodium chloride melts at 801 °C and boils at 1413 °C. (As a comparison, the molecular compound water melts at 0 °C and boils at 100 °C.) In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). When molten, however, it can conduct electricity because its ions are able to move freely through the liquid (Figure \(\PageIndex{3}\); Video \(\PageIndex{3}\)).
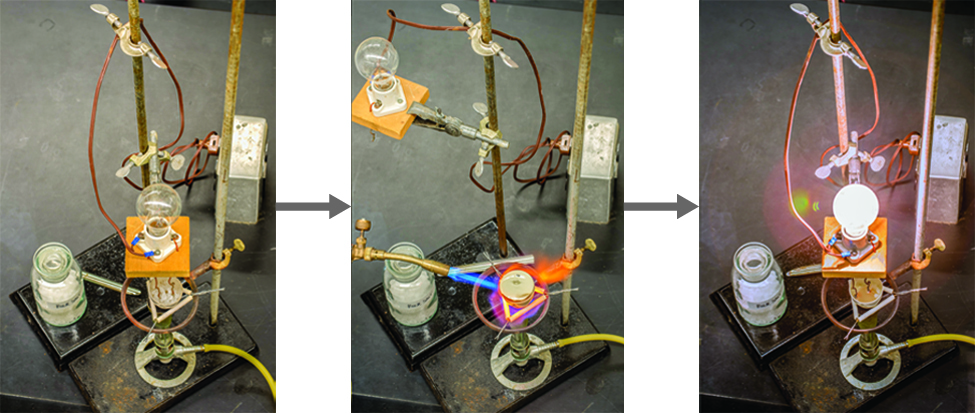
Figure \(\PageIndex{2}\): Sodium chloride melts at 801 °C and conducts electricity when molten. (credit: modification of work by Mark Blaser and Matt Evans)
Video \(\PageIndex{3}\): Watch this video to see a mixture of salts melt and conduct electricity.
In every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. Thus, ionic compounds are electrically neutral overall, even though they contain positive and negative ions. We can use this observation to help us write the formula of an ionic compound. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal.
Example \(\PageIndex{3}\): Predicting the Formula of an Ionic Compound
The gemstone sapphire (Figure \(\PageIndex{4}\)) is mostly a compound of aluminum and oxygen that contains aluminum cations, Al3+, and oxygen anions, O2−. What is the formula of this compound?

Figure \(\PageIndex{4}\): Although pure aluminum oxide is colorless, trace amounts of iron and titanium give blue sapphire its characteristic color. (credit: modification of work by Stanislav Doronenko)
Solution Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative charges. The formula would be Al2O3.
Exercise \(\PageIndex{3}\)
Predict the formula of the ionic compound formed between the sodium cation, Na+, and the sulfide anion, S2−.
- Answer
-
Na2S
Molecular (Covalent) Compounds
Video \(\PageIndex{4}\): What are covalent bonds?
Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Covalent bonding is an important and extensive concept in chemistry, and it will be treated in considerable detail in a later chapter of this text. We can often identify molecular compounds on the basis of their physical properties. Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist.
Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Thus, the periodic table can help us recognize many of the compounds that are covalent. While we can use the positions of a compound’s elements in the periodic table to predict whether it is ionic or covalent at this point in our study of chemistry, you should be aware that this is a very simplistic approach that does not account for a number of interesting exceptions. Shades of gray exist between ionic and molecular compounds, and you’ll learn more about those later.
Example \(\PageIndex{2}\): Predicting the Type of Bonding in Compounds
Predict whether the following compounds are ionic or molecular:
- KI, the compound used as a source of iodine in table salt
- H2O2, the bleach and disinfectant hydrogen peroxide
- CHCl3, the anesthetic chloroform
- Li2CO3, a source of lithium in antidepressants
Solution
- Potassium (group 1) is a metal, and iodine (group 17) is a nonmetal; KI is predicted to be ionic.
- Hydrogen (group 1) is a nonmetal, and oxygen (group 16) is a nonmetal; H2O2 is predicted to be molecular.
- Carbon (group 14) is a nonmetal, hydrogen (group 1) is a nonmetal, and chlorine (group 17) is a nonmetal; CHCl3 is predicted to be molecular.
- Lithium (group 1) is a metal, and carbonate is a polyatomic ion; Li2CO3 is predicted to be ionic.
Exercise \(\PageIndex{2}\)
Using the periodic table, predict whether the following compounds are ionic or covalent:
- SO2
- CaF2
- N2H4
- Al2(SO4)3
- Answer a
-
molecular
- Answer b
-
ionic
- Answer c
-
molecular
- Answer d
-
ionic
Polyatomic Ions
The ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one atom. Polyatomic ions are are composed of more than one atom, united chemically. For example, the ammonium ion consists of one nitrogen atoms and four hydrogen atoms linked together via covalent bonds, and together they comprise a single ion with a \(1+\) charge and a formula of \(\ce{NH_4^+}\). The carbonate ion consists of one carbon atom and three oxygen atoms and carries an overall charge of \(2-\). The formula of the carbonate ion is \(\ce{CO_3^{2-}}\). The atoms of a polyatomic ion are covalently bonded together and so the entire ion behaves as a single unit.
Some of the more important polyatomic ions are listed in Table \(\PageIndex{1}\). Because you will use them repeatedly, they will soon become familiar.
Note that there is a system for naming some polyatomic ions: Oxyanions are polyatomic ions that contain one or more oxygen atoms; -ate and -ite are suffixes designating polyatomic ions containing more or fewer oxygen atoms. Per- (short for “hyper”) and hypo- (meaning “under”) are prefixes meaning more oxygen atoms than -ate and fewer oxygen atoms than -ite, respectively. For example, perchlorate is \(\ce{ClO4-}\), chlorate is \(\ce{ClO3-}\), chlorite is \(\ce{ClO2-}\) and hypochlorite is ClO−. Unfortunately, the number of oxygen atoms corresponding to a given suffix or prefix is not consistent; for example, nitrate is \(\ce{NO3-}\) while sulfate is \(\ce{SO4^{2-}}\).
| Name | Formula | Related Acid | Formula |
|---|---|---|---|
| ammonium | \(\ce{NH4+}\) | ||
| hydronium | \(\ce{H_3O^+}\) | ||
| oxide | \(\ce{O^{2-}}\) | ||
| peroxide | \(\ce{O_2^{2-}}\) | ||
| hydroxide | \(\ce{OH^-}\) | ||
| acetate | \(\ce{CH_3COO^-}\) | acetic acid | \(\ce{CH_3COOH}\) |
| cyanide | \(\ce{CN^-}\) | hydrocyanic acid | \(\ce{HCN}\) |
| azide | \(\ce{N_3^-}\) | hydrazoic acid | \(\ce{HN_3}\) |
| carbonate | \(\ce{CO_3^{2-}}\) | carbonic acid | \(\ce{H_2CO_3}\) |
| bicarbonate | \(\ce{HCO_3^-}\) | ||
| nitrate | \(\ce{NO_3^-}\) | nitric acid | \(\ce{HNO_3}\) |
| nitrite | \(\ce{NO_2^-}\) | nitrous acid | \(\ce{HNO_2}\) |
| sulfate | \(\ce{SO_4^{2-}}\) | sulfuric acid | \(\ce{H_2SO_4}\) |
| hydrogen sulfate | \(\ce{HSO_4^-}\) | ||
| sulfite | \(\ce{SO_3^{2-}}\) | sulfurous acid | \(\ce{H_2SO_3}\) |
| hydrogen sulfite | \(\ce{HSO_3^-}\) | ||
| phosphate | \(\ce{PO_4^{3-}}\) | phosphoric acid | \(\ce{H_3PO_4}\) |
| hydrogen phosphate | \(\ce{HPO_4^{2-}}\) | ||
| dihydrogen phosphate | \(\ce{H_2PO_4^-}\) | ||
| perchlorate | \(\ce{ClO_4^-}\) | perchloric acid | \(\ce{HClO_4}\) |
| chlorate | \(\ce{ClO_3^-}\) | chloric acid | \(\ce{HClO_3}\) |
| chlorite | \(\ce{ClO_2^-}\) | chlorous acid | \(\ce{HClO_2}\) |
| hypochlorite | \(\ce{ClO^-}\) | hypochlorous acid | \(\ce{HClO}\) |
| chromate | \(\ce{CrO_4^{2-}}\) | chromic acid | \(\ce{H_2CrO_4}\) |
| dichromate | \(\ce{Cr_2O_7^{2-}}\) | dichromic acid | \(\ce{H_2Cr_2O7}\) |
| permanganate | \(\ce{MnO_4^-}\) | permanganic acid | \(\ce{HMnO_4}\) |
Compounds Containing Polyatomic Ions
Polyatomic ions will form ionic bonds with monoatomic or polyatomic ions to make ionic compounds. For example, ammonium nitrate is an ionic compound composed of the polyatomic cation ammonium \(\ce{NH_4^+}\) and the polyatomic anion nitrate \(\ce {NO_3^-}\). Because ammonium carries a \(1+\) charge and nitrate carries a \(1-\) charge, the two ions will be in a 1:1 ratio and the ionic compound will be electrically neutral; the formula for ammonium nitrate is \(\ce{NH_4}{NO_3}\).
An example of an ionic compound formed between a monoatomic and a polyatomic ion is lithium dichromate: \(\ce{Li_2}{Cr_2O_7}\). Note that the lithium cation, \(\ce{Li_2^+}\), carries a \(1+\) charge and the dichromate anion, \(\ce {Cr_2O_7^2}\), carries a \(2-\) charge, therefore in order for the formula to be neutral, there must be two lithium ions for every one dichromate ion. Use the table of polyatomic ioncs (Table 4.1.1) when predicting formulas for, or naming ionic compounds that include a polyatomic ion.
Example \(\PageIndex{3}\): Predicting the Formula of a Compound with a Polyatomic Anion
Baking powder contains calcium dihydrogen phosphate, an ionic compound composed of the ions Ca2+ and \(\ce{H2PO4-}\). What is the formula of this compound?
Solution
The positive and negative charges must balance, and this ionic compound must be electrically neutral. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. This requires a ratio of one Ca2+ ion to two \(\ce{H2PO4-}\) ions. We designate this by enclosing the formula for the dihydrogen phosphate ion in parentheses and adding a subscript 2. The formula is Ca(H2PO4)2.
Exercise \(\PageIndex{3}\)
Predict the formula of the ionic compound formed between the lithium ion and the peroxide ion, \(\ce{O2^2-}\) (Hint: Use the periodic table to predict the sign and the charge on the lithium ion.)
- Answer
-
Li2O2
Summary
The nature of the attractive forces that hold atoms or ions together within a compound is the basis for classifying chemical bonding. When electrons are transferred and ions form, ionic bonds result. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this case, cations and anions). When electrons are “shared” and molecules form, covalent bonds result. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Compounds are classified as ionic or molecular (covalent) on the basis of the bonds present in them.
Metals (particularly those in groups 1 and 2) tend to lose the number of electrons that would leave them with the same number of electrons as in the preceding noble gas in the periodic table. By this means, a positively charged ion is formed. Similarly, nonmetals (especially those in groups 16 and 17, and, to a lesser extent, those in Group 15) can gain the number of electrons needed to provide atoms with the same number of electrons as in the next noble gas in the periodic table. Thus, nonmetals tend to form negative ions. Positively charged ions are called cations, and negatively charged ions are called anions. Ions can be either monatomic (containing only one atom) or polyatomic (containing more than one atom).
Compounds that contain ions are called ionic compounds. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds. Covalent compounds usually form from two or more nonmetals.
How Atoms Bond
Video \(\PageIndex{6}\): A summary of chemical bonds.
Glossary
- binary compound
- compound containing two different elements.
- covalent bond
- attractive force between the nuclei of a molecule’s atoms and pairs of electrons between the atoms
- covalent compound
- (also, molecular compound) composed of molecules formed by atoms of two or more different elements
- ionic bond
- electrostatic forces of attraction between the oppositely charged ions of an ionic compound
- ionic compound
- compound composed of cations and anions combined in ratios, yielding an electrically neutral substance
- molecular compound
- (also, covalent compound) composed of molecules formed by atoms of two or more different elements
- monatomic ion
- ion composed of a single atom
- polyatomic ion
- ion composed of more than one atom
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Institute of Technology
- TED-Ed’s commitment to creating lessons worth sharing is an extension of TED’s mission of spreading great ideas. Within TED-Ed’s growing library of TED-Ed animations, you will find carefully curated educational videos, many of which represent collaborations between talented educators and animators nominated through the TED-Ed website.
- Fuse School, Open Educational Resource free of charge, under a Creative Commons License: Attribution-NonCommercial CC BY-NC (View License Deed: https://creativecommons.org/licenses/by-nc/4.0/)
Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook

