14.6: Cycloaddition reactions
- Page ID
- 434920
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Diels-Alder Reactions:
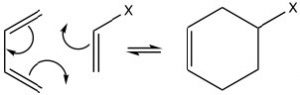
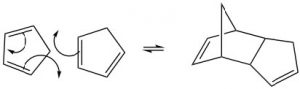
Cylcoaddition reactions are addition reactions that form a ring. The best-known cycloaddition reaction is the Diels-Alder reaction. It typically occurs between a conjugated diene and another alkene known as the dienophile. This reaction is very useful in synthesis because it forms two new \(\mathrm{C-C}\) bonds at the same time. It is also stereospecific and regiospecific. The simplest diene that can participate in a Diels-Alder is butadiene. Typically, butadiene tends to exist in the more stable s-trans[10] conformation—however, there is an equilibrium concentration of the s-cis conformation through which the reaction can occur. In fact, cyclopentadiene, in which the diene is permanently fused in the s-trans conformation, reacts rapidly with itself (it can be both diene and dienophile) in a reversible reaction.
The “dienophile” can be a simple alkene, but the presence of an electron-withdrawing group (such as a carbonyl group) on the double bond improves yields. We can envision the reaction as taking place in a concerted fashion. Again, the reaction is reversible, and the reverse reaction is known as a retro Diels-Alder. We can use our knowledge of thermodynamics to predict the most appropriate conditions for the reaction. Recall that the extent of any reaction can be predicted from the Gibbs free-energy change \(\Delta \mathrm{G} = \Delta \mathrm{H} – T \Delta \mathrm{S}\). Since the reaction produces two new \(\mathrm{C-C}\) single bonds and one new \(\mathrm{C-C}\) pi bond while breaking two \(\mathrm{C-C}\) pi bonds, we can assume that the enthalpy change for this reaction is negative (bond formation releases energy and bond-breaking uses energy). Therefore, the \(\Delta \mathrm{H}\) term is always favorable. We can also predict the sign of the entropy change for the system; since we are producing one molecule from two, we would expect \(\Delta \mathrm{S}\) to be negative also. The entropy term is unfavorable. From this analysis, we can see that the temperature at which the reaction is carried out is crucial. High temperatures would favor the reverse reaction. Therefore Diels-Alder reactions are typically run at fairly moderate temperatures that are between room temperature and \(150^{\circ} \mathrm{C}\)).
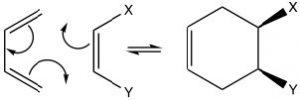
The possibilities for Diels-Alder reactions are quite extensive and since this is a concerted reaction, any stereochemistry in the starting materials is conserved in the products. For example, if the dienophile has cis or trans stereochemistry, this is conserved in the product. The cis dienophile gives the cis product (and vice versa for the trans).
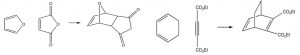
Some examples of Diels-Alder reactions are given here.
While we can draw mechanistic arrows for cycloaddition reactions such as Diels-Alder, they are best understood by using molecular orbital theory. In this treatment, we can consider the reaction as taking place between the highest occupied molecular orbital (HOMO) of the diene and the lowest unoccupied molecular orbital (LUMO) of the dienophile.
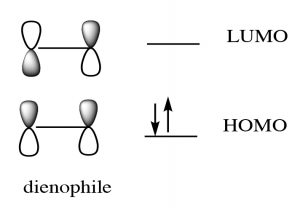
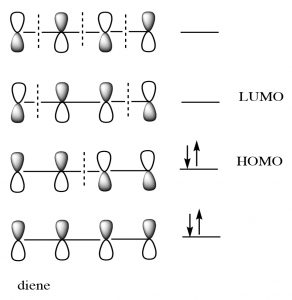
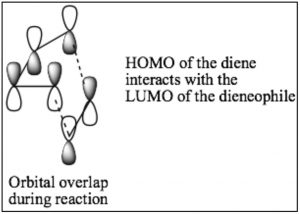
For our purposes, this treatment is too complex, but if you go on to further studies of cycloaddition reactions, MO theory will be the approach that will allow you to predict the outcome of many different reactions.
If a fused ring is formed during the reaction, there are two possibilities for the orientation of the substituents on the diene as shown below: exo and endo.
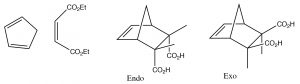
Because of the possibility of a stabilizing interaction in the endo position, (the pi system of the carboxylic acid can interact with the pi system of the diene), the endo product is usually produced.
As noted earlier, there are other types of cycloaddition reactions. All of the common reactions that occur simply by heating up the starting materials involve the cyclic movement of six electrons. The transition states for these reactions all involve molecular orbitals that extend throughout the system; these orbitals have considerably lower energy than one might expect. In some ways, this is analogous to the 6 pi electrons of aromatic systems, which are also stabilized in lower energy molecular orbitals.
Interestingly, these reactions do not occur in the same way if they are initiated by electromagnetic radiation (that is, if we shine light on them rather than heat them up). In this case the electrons that participate in the reaction are actually in higher energy orbitals (the electron absorbs a photon and is promoted to a higher energy level). Absorbing light leads to a completely different set of reactions and outcomes, something that could be explored in subsequent organic chemistry courses. There is, however, one particularly interesting (and biologically relevant) photochemically induced reaction, namely the reaction of adjacent thymine bases within a DNA molecule. Upon the absorption of a UV photon, such adjacent thymines can undergo a cycloaddition reaction that results in formation of a thymine dimer.
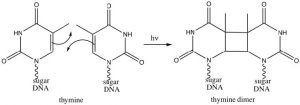
The presence of a thymine dimer results in conformational changes in the DNA that, unrepaired, can lead to mutations during DNA replication. Thymine dimers are recognized and repaired via two distinct cellular-repair mechanisms. Unrepaired damage can lead to skin cancer (a range of carcinomas and melanomas). This is one reason to limit skin exposure to the sun.

