What Is Electrophilic Hydration?
Electrophilic hydration is the addition of hydrogen and a hydroxyl group across the two carbons of a double bond. Electrophilic hydration is the reverse of dehydration of alcohols and so begins the circular nature of organic chemistry. Alcohols can be dehydrated to form alkenes and alkenes can undergo electrophilic addition reactions to form alcohols. Electrophilic hydrogen is essentially a proton: a hydrogen atom stripped of its electrons. Electrophilic hydrogen is commonly used to help break double bonds or restore catalysts.
Electrophilic hydration of alkenes has practical applications in making alcohols for fuels and reagents for other reactions. The basic reaction under certain temperatures (given below) is the following:
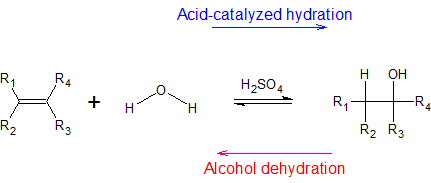
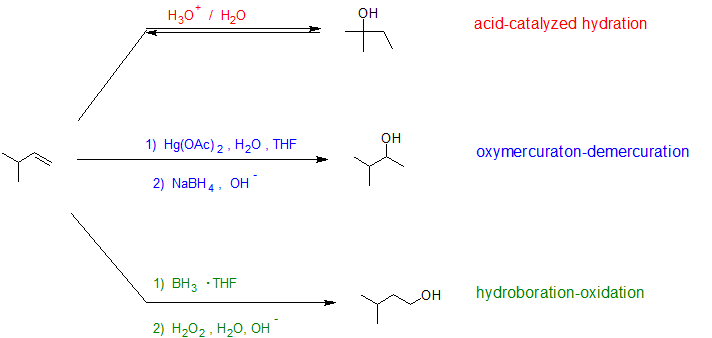
In later sections, we will learn that mercury (II) sulfate and borane are also electrophiles that can react with alkenes to form hydration products. Each reaction pathway has its own regio- and stereochemical considerations. In the example below, we see that the same alkene produces different hydration products depending on the hydration pathway.
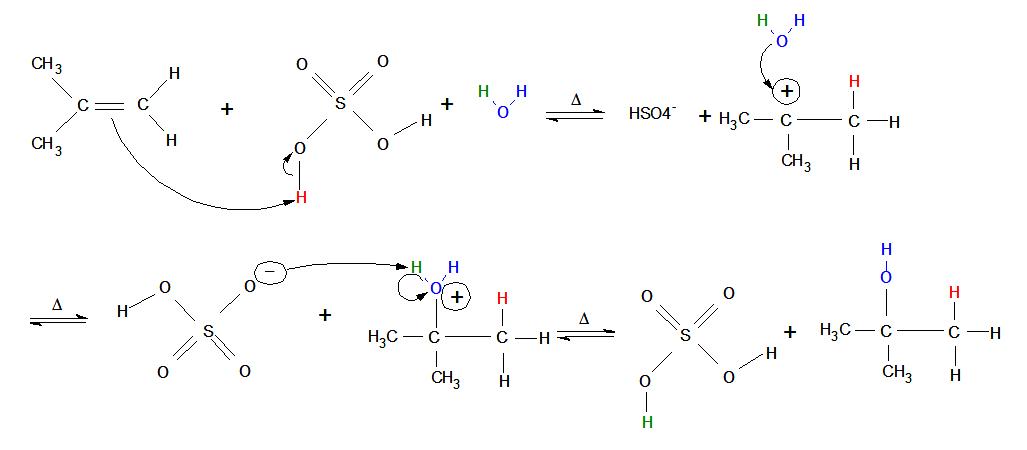
Mechanism for Acid-catalyzed Hydration of Alkenes
Temperatures for Types of Alcohol Synthesis
Heat is used to catalyze electrophilic hydration; because the reaction is in equilibrium with the dehydration of an alcohol, which requires higher temperatures to form an alkene, lower temperatures are required to form an alcohol. The exact temperatures used are highly variable and depend on the product being formed.
- Primary Alcohol: Less than 170ºC
- Secondary Alcohol: Less than 100ºC
- Tertiary Alcohol: Less than 25ºC
But...Why Does Electrophilic Hydration Work?
- An alkene placed in an aqueous non-nucleophilic strong acid immediately "reaches out" with its double bond and attacks one of the acid's hydrogen atoms (meanwhile, the bond between oxygen and hydrogen performs heterolytic cleavage toward the oxygen—in other words, both electrons from the oxygen/hydrogen single bond move onto the oxygen atom).
- A carbocation is formed on the original alkene (now alkane) in the more-substituted position, where the oxygen end of water attacks with its 4 non-bonded valence electrons (oxygen has 6 total valence electrons because it is found in Group 6 on the periodic table and the second row down: two electrons in a 2s-orbital and four in 2p-orbitals. Oxygen donates one valence electron to each bond it forms, leaving four 4 non-bonded valence electrons).
- After the blue oxygen atom forms its third bond with the more-substituted carbon, it develops a positive charge (3 bonds and 2 valence electrons give the blue oxygen atom a formal charge of +1).
- The bond between the green hydrogen and the blue oxygen undergoes heterolytic cleavage, and both the electrons from the bond move onto the blue oxygen. The now negatively-charged strong acid picks up the green electrophilic hydrogen.
- Now that the reaction is complete, the non-nucleophilic strong acid is regenerated as a catalyst and an alcohol forms on the most substituted carbon of the current alkane. At lower temperatures, more alcohol product can be formed.
What is Regiochemistry and How Does It Apply?
Regiochemistry deals with where the substituent bonds on the product. Zaitsev's and Markovnikov's rules address regiochemistry, but Zaitsev's rule applies when synthesizing an alkene while Markovnikov's rule describes where the substituent bonds onto the product. In the case of electrophilic hydration, Markovnikov's rule is the only rule that directly applies. See the following for an in-depth explanation of regiochemistry Markovnikov explanation: Radical Additions--Anti-Markovnikov Product Formation
In the mechanism for a 3º alcohol shown above, the red H is added to the least-substituted carbon connected to the nucleophilic double bonds (it has less carbons attached to it). This means that the carbocation forms on the 3º carbon, causing it to be highly stabilized by hyperconjugation—electrons in nearby sigma (single) bonds help fill the empty p-orbital of the carbocation, which lessens the positive charge. More substitution on a carbon means more sigma bonds are available to "help out" (by using overlap) with the positive charge, which creates greater carbocation stability. In other words, carbocations form on the most substituted carbon connected to the double bond. Carbocations are also stabilized by resonance, but resonance is not a large factor in this case because any carbon-carbon double bonds are used to initiate the reaction, and other double bonded molecules can cause a completely different reaction. If the carbocation does originally form on the less substituted part of the alkene, carbocation rearrangements occur to form more substituted products.
Carbocation Rearrangements - a review
- Hydride shifts: a hydrogen atom bonded to a carbon atom next to the carbocation leaves that carbon to bond with the carbocation (after the hydrogen has taken both electrons from the single bond, it is known as a hydride). This changes the once neighboring carbon to a carbocation, and the former carbocation becomes a neighboring carbon atom.
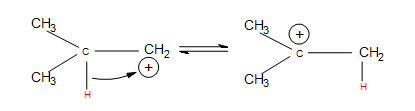
In a more complex case, when alkenes undergo hydration, we also observe hydride shift. Below is the reaction of 3-methyl-1-butene with H3O+ that furnishes to make 2-methyl-2-butanol:
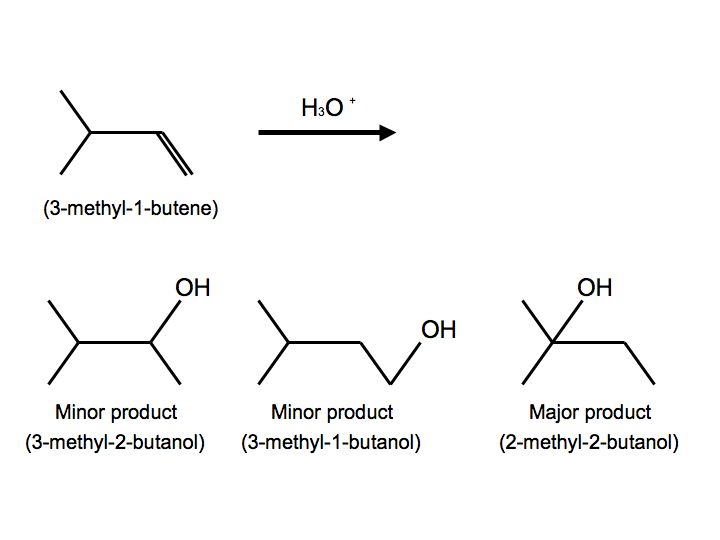.jpg?revision=1&size=bestfit&width=456&height=340)
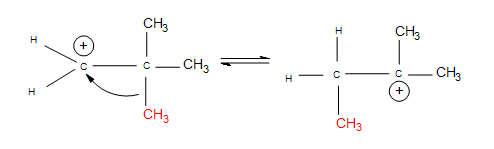
Once again, we see multiple products. In this case, however, we see two minor products and one major product. We observe the major product because the -OH substitutent is attached to the more substituted carbon. When the reactant undergoes hydration, the proton attaches to carbon #2. The carbocation is therefore on carbon #2. Hydride shift now occurs when the hydrogen on the adjacent carbon formally switch places with the carbocation. The carbocation is now ready to be attacked by H2O to furnish an alkyloxonium ion because of stability and hyperconjugation. The final step can be observed by another water molecule attacking the proton on the alkyloxonium ion to furnish an alcohol. We see this mechanism below:
.jpg?revision=1&size=bestfit&width=512&height=383)
- Alkyl shifts: if no hydrogen atoms are available for a hydride shift, an entire methyl group performs the same shift.
The nucleophile attacks the positive charge formed on the most substituted carbon connected to the double bond, because the nucleophile is seeking that positive charge. In the mechanism for a 3º alcohol shown above, water is the nucleophile. When the green H is removed from the water molecule, the alcohol attached to the most substituted carbon. Hence, electrophilic hydration follows Markovnikov's rule.
Stereochemistry of Acid-catalyzed Hydration
Stereochemistry deals with how the substituent bonds on the product directionally. Dashes and wedges denote stereochemistry by showing whether the molecule or atom is going into or out of the plane of the board. Whenever the bond is a simple single straight line, the molecule that is bonded is equally likely to be found going into the plane of the board as it is out of the plane of the board. This indicates that the product is a racemic mixture.
There is no stereochemical control in acid-catalyzed hydration reactions. The carbocation intermediate has the trigonal planar geometry of sp2 hybridization which allows the subsequent reaction with water from either orientation.
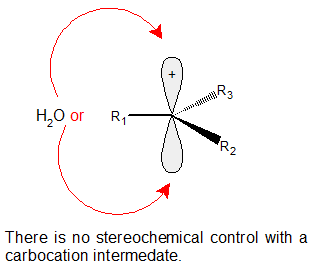
Electrophilic hydration adopts a stereochemistry wherein the substituent is equally likely to bond pointing into the plane of the board as it is pointing out of the plane of the board. The 3º alcohol product could look like either of the following products:
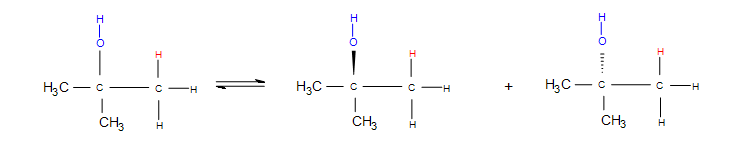
Note: Whenever a straight line is used along with dashes and wedges on the same molecule, it could be denoting that the straight line bond is in the same plane as the board. Practice with a molecular model kit and attempting the practice problems at the end can help eliminate any ambiguity.
Is this a Reversible Synthesis?
Electrophilic hydration is reversible because an alkene in water is in equilibrium with the alcohol product. To sway the equilibrium one way or another, the temperature or the concentration of the non-nucleophilic strong acid can be changed. For example:
- Less sulfuric or phosphoric acid and an excess of water help synthesize more alcohol product.
- Lower temperatures help synthesize more alcohol product.
Is There a Better Way to Add Water to Synthesize an Alcohol From an Alkene?
A more efficient pathway does exist: see Oxymercuration - Demercuration: A Special Electrophilic Addition. Oxymercuration does not allow for rearrangements, but it does require the use of mercury, which is highly toxic. Detractions for using electrophilic hydration to make alcohols include:
- Allowing for carbocation rearrangements
- Poor yields due to the reactants and products being in equilibrium
- Allowing for product mixtures (such as an (R)-enantiomer and an (S)-enantiomer)
- Using sulfuric or phosphoric acid
Exercise
1. Draw the bond-line structure for the major product of each reaction.
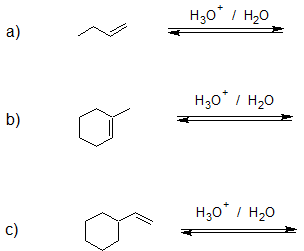
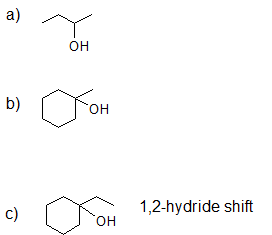
- Answer
-
1.






.jpg?revision=1&size=bestfit&width=456&height=340)
.jpg?revision=1&size=bestfit&width=512&height=383)




