1.7: The Importance of Ions
- Page ID
- 283312
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Chemists are frequently concerned with the movement of electrons. When an atom gains or loses electrons, it becomes a charged species called an ion. When this occurs, the nucleus is not altered. For atoms that lose electrons, an overall positive charge will result (#protons > #electrons). Atoms that form these types of ions are called cations. Metal atoms (located on the left side of the periodic table) always lose electrons to become cations.
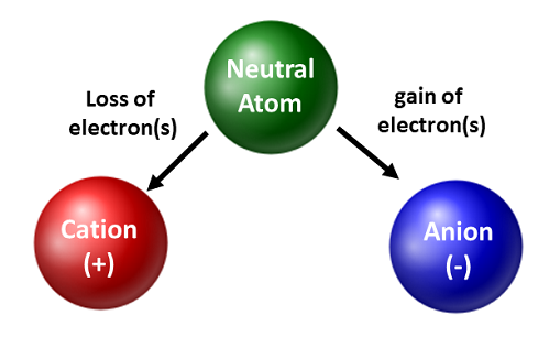
Unlike metal atoms, nonmetals will gain electrons to become anions. These types of ions have an overall negative charge (#electrons > #protons). With the exception of the noble gases, all atoms on the periodic table will lose or gain electrons to achieve electronic stability. Different types of bonding occur when atoms lose, gain, or share electrons. These types of atomic connections will be further discussed in the next section and again later in the course.
Interactive: Building an Atom
Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
At this point, you should be able to calculate all subatomic particles when given a specific ion charge. In addition, you should be able to classify ions as cations or anions.
The ions of main group elements tend to have charges that follow patterns as shown in the figure below. This is based on the number of valence electrons an atom has. Remember that atoms of hydrogen and the alkali metals each have one valence electron. That valence electron can be lost resulting in a cation with a charge of +1. Alkaline earth metals each have two valence electrons and can form cations with a +2 charge. Nonmetals in groups 15-17 have five to seven valence electrons and can gain the 1-3 valence electrons that they need to achieve eight valence electrons, a particularly stable configuration. Remember that because electrons are negative, an atom that gains extra electrons becomes negative overall.
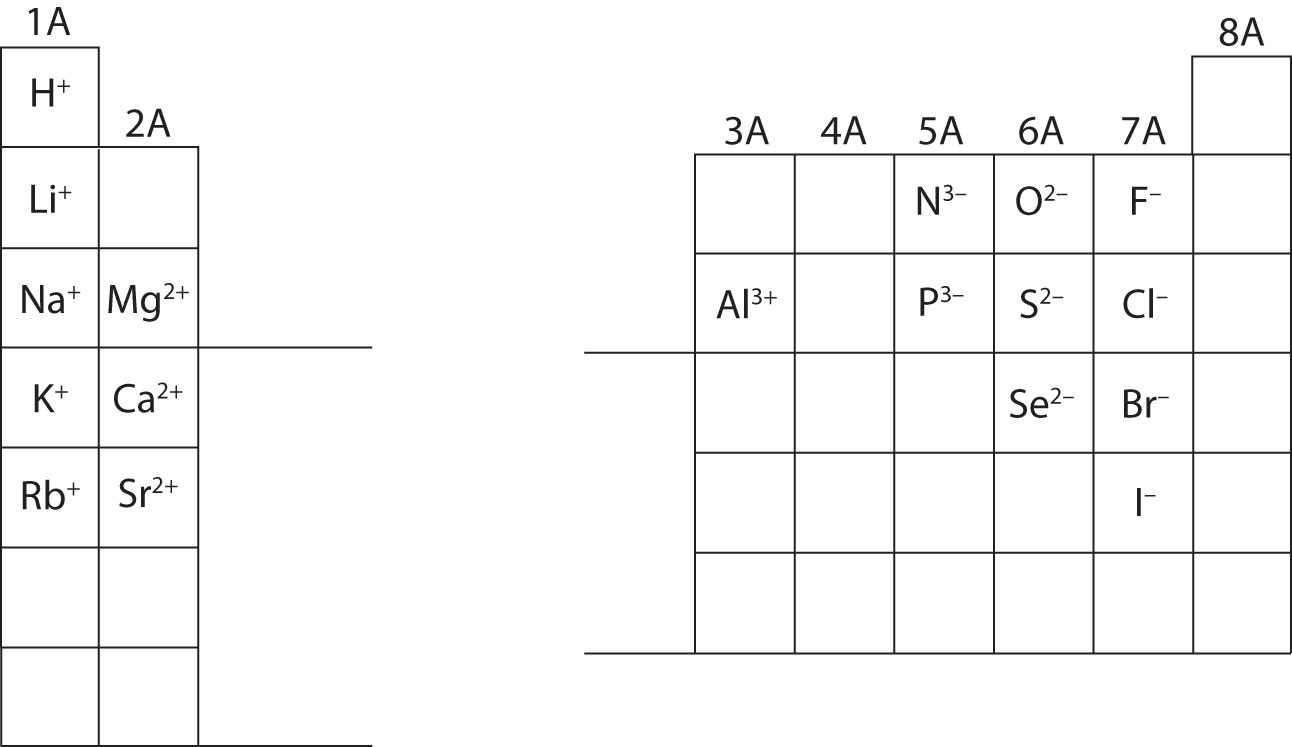
Most transition metals, as well as the metals at the bottom of groups 13-15, can form more than one ion. For example, iron can be found as ions with a +2 charge or a +3 charge. These are called iron(II) ions and iron(III) ions, respectively. Other elements have different possible charges such as nickel(I) and nickel(II) or lead(II) and lead(IV). You don't need to memorize all of these possibilities! Instead, notice that the name of the ion gives the charge as a Roman numeral.
The noble gases in group 8 already have eight valence electrons, so they do not readily form ions.
Example \(\PageIndex{1}\): Ion Classification
Calculate the subatomic particles for the species below using the information in Figure \(\PageIndex{2}\). Label each as being an atom, cation, or anion. Refer to the periodic table for masses, atomic numbers, and specific ion charges.
- Aluminum ion
- Zirconium atom
- Sulfur ion
Solutions
- There are 13 protons, 14 neutrons, and 10 electrons in the aluminum ion. This ion is positively charged which means it has lost electrons and forms a cation.
- There are 40 protons, 51 neutrons, and 40 electrons in the zirconium atom. This is an atom which has no overall charge.
- There are 16 protons, 16 neutrons, and 18 electrons in the sulfur ion. This ion is negatively charged which means it has gained electrons and forms and anion.
Contributors


