Water treatment is` a process of making water suitable for its application or returning its natural state. Thus, water treatment required before and after its application. The required treatment depends on the application. For example, treatment of greywater (from bath, dish and wash water) differs from the black water (from flush toilets). Composting toilet is not allowed in urban dwelling. Yet, composting toilets are used in a 30,000-square-foot office complex at the Institute of Asian Research, University of British Columbia.
Water treatment involves science, engineering, business, and art. The treatment may include mechanical, physical, biological, and chemical methods. As with any technology, science is the foundation, and engineering makes sure that the technology works as designed. The appearance and application of water is an art.
In terms of business, RGF Environmental, Water Energy Technologies, Aquasana Store, Vitech, Recalyx Industrial SDN BHD and PACE Chemicals ltd are some of many companies that offer various processes for water treatment. Millipore, a Fisher Scientific partner, offers many lines of products to produce ultrapure water, using a combination of active charcoal membranes, and reverse osmosis filter. Internet sites of these companies offer useful information regarding water.
An environmental scientist or consultant matches the service provider, modify if necessary, with the requirement.
General Wastewater Treatment
Water is a renewable resource. All water treatments involve the removal of solids, bacteria, algae, plants, inorganic compounds, and organic compounds. Removal of solids is usually done by filtration and sediment. Bacteria digestion is an important process to remove harmful pollutants. Converting used water into environmentally acceptable water or even drinking water is wastewater treatment
Water in the Great Lakes Region is an organization dealing with the water resources. Ontario Clean Water Agency (OCWA) is a provincial Crown corporation in business to provide environmentally responsible and cost-efficient water and wastewater services. It currently operates more than 400 facilities for 200 municipalities. This web site provides information on water and water treatment.
In April 1993, 403,000 people in Milwaukee were ill as a result of cryptosporidium contaimination of water due to spring run off. This outbreak caused the more stringent regulations to be implemented in the public dringking water system. The measures were aimed at removing cryptosporidium.
In May 2000, due to torrential downpour surface water got into shallow wells in a small town Walkerton, Ontario, Canada. On May 17, some residents complained of fever, bloody diarrhea and vomiting. This was know as the Walkerton E. Coli Outbreak. Nearly half of the population of the town fell ill, and several people died due to the E. Coli O157:H7 infection. A public inquiry recommended many measures to prevent similar outbreaks. These measures were aimed at eliminating E. Coli.
Sewage Treatment
As a general discussion, let us look at a typical process in sewage treatment. A flow diagram for a general sewage treatment plant from Water Education, Department of Computer Science, University of Exeter, U.K., is shown below:
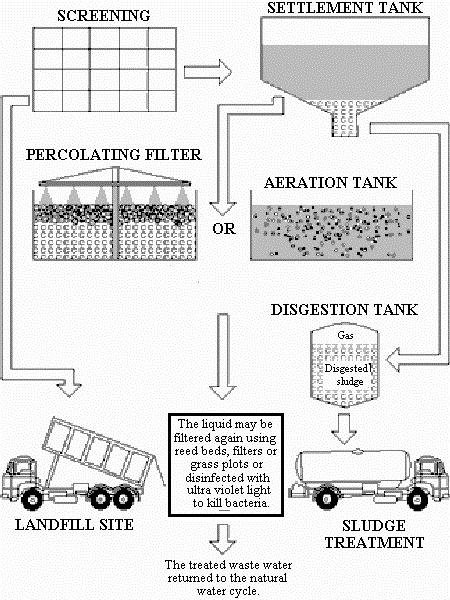
Sewage is SCREENED to remove large solid chunks, which are disposed in LANDFILL SITE. It flows over to the SETTLEMENT TANK to let the fine particles to settle. The settlement is called the activated SLUDGE. The supernatant is then PERCOLATING FILTERED and/or AERATED. The water can be filtered again, and then disinfected (chlorinated in most cases). When there is no other complication, the water is returned to nature back to the ecological cycle.
The SLUDGE removed from the settlement is composed of living biological material. A portion of it may be returned to the AERATION TANK, but the raw SLUDGE is digested by both microorganism. Anaerobic (without oxygen) and aerobic (with air) bacteria digestions are used. At the digestion stage, carbon dioxide, ammonia, and methane gases are evolved. Volume of the digested sludge is reduced, and it is acceptable as a fertilizer supplement in farming.
Wastewater Treatment
Although the sewage water may be discharged back to the ecological system after AERATED DIGESTION and PERCOLATING FILTRATION, but in some cases, further treatment is required. Some general consideration of water treatment is given below.
A rather recent book, Chemistry of Water Treatment by S.D. Faust and O.M. Aly, 2nd Ed. (1998) [TD433 F38 1998], addresses the problem of quality natural and treated water.
The first three chapters discuss the criteria and standards for drinking water quality, organic compounds in waters, taste and order of water. Understandably, the standards change over the years. So are the standards of treated waters. Guidelines are available from government agencies such as Environment Canada which is equivalent to U.S. Public Health Service and the Environment Protection Agency (EPA). We have talked about drinking water in Water Biology.
Next seven chapters deal with the removal of the following:
- organics and inorganics by activated carbon
- particulate matter by coagulation
- particulate matter by filtration and sedimentation
- hardness and other scale-forming substances
- inorganic contaminants
- corrosive substances
- pathogenic (disease producing) bacteria, viruses, and protozoans (microorganisms).
There is a chapter dealing with aeration
These items cover the chemistry, biology, and physics involved in the treatment of water. Some of these topics have been discussed in chemistry of water, physical properties of water, biology of water, and natural water. Introductions are going to be given to some selected topics below.
Treatment by Activated Carbon
Treatment by activated carbon is mostly due to adsorption or absorption. When a chemical species is adhered to the surface of a solid, it is an adsorption. When partial chemical bonds are formed between adsorbed species or when the absorbate got into the channels of the solids, we call it absorption. However, these two terms are often used to mean the same, because to distinguish one from type from the other is very difficult.
Application of activated charcoal for the removal of undesirable order and taste in drinking water has been recognized at the dawn of civilization. Using bone char and charred vegetation, gravel, and sand for the filtration of water for domestic application has been practised for thousands of years. Active research and production of activated charcoal was accelerated during the two world wars. The use of poison gas prompted the development of masks. They are still in use today.
Charcoal absorbs many substances, ranging from colored organic particulates to inorganic metal ions. Charcoal has been used to remove the colour of raw sugar from various sources.
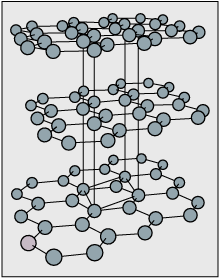 Charcoal consists of microcrystallites of graphite. The particles are so small in charcoal that they were considered amorphous. The crystal structure of graphite consists of layers of hexagonal networks, stacked on top of each other. Today, making activated carbon is a new and widely varied industry. Other molecules attach themselves to the porous surface and dangling carbons in these microcrystallites.
Charcoal consists of microcrystallites of graphite. The particles are so small in charcoal that they were considered amorphous. The crystal structure of graphite consists of layers of hexagonal networks, stacked on top of each other. Today, making activated carbon is a new and widely varied industry. Other molecules attach themselves to the porous surface and dangling carbons in these microcrystallites.
Carbon containing substances are charred at less than 900 K to produce carbon in the manufacture of activated carbon. However, the carbon is activated at 1200 K using oxidizing agent to selectively oxidize portions of the char to produce pores in the material. Because of the special process to produce used, these materials with high surface to mass ratio, they are called activated carbon rather than activated charcoal. Factors affecting the absorption are particle size, surface area, pore structure, acidity (pH), temperature, and the nature of the material to be absorbed. Usually, adsorption (absorption) equilibria and rate of adsorption must be considered for effective applications.
Coagulation, Flucculation and Sedimentation
Natural and wastewater containing small particulates. They are suspended in water forming a colloid. These particles carry the same charges, and repulsion prevents them from combining into larger particulates to settle. Thus, some chemical and physical techniques are applied to help them settle. The phenomenon is known as coagulation. A well known method is the addition of electrolyte. Charged particulates combine with ions neutralizing the charges. The neutral particulates combine to form larger particles, and finally settle down.
Another method is to use high-molecular-weight material to attract or trap the particulates and settle down together. Such a process is called flocculation. Starch and multiply charged ions are often used.
Historically, dirty water is cleaned by treating with alum, Al2(SO4)3.12 H2O, and lime, Ca(OH)2. These electrolytes cause the pH of the water to change due to the following reactions:
Al2(SO4)3.12 H2O, -> Al(aq)3+ + 3 SO4(aq)2- + 12 H2O
SO4(aq)2- + H2O -> HSO4(aq)- + OH- (causing pH change)
Ca(OH)2 -> Ca(aq)2+ + 2 OH- (causing pH change)
The slightly basic water causes Al(OH)3, Fe(OH)3 and Fe(OH)2 to precipitate, bringing the small particulates with them and the water becomes clear. Some records have been found that Egyptians and Romans used these techniques as early as 2000 BC.
Suspension of iron oxide particulates and humic organic matter in water gives water the yellow muddy appearance. Both iron oxide particulates and organic matter can be removed from coagulation and flocculation. The description given here is oversimplified, and many more techniques have been applied in the treatment of water. Coagulation is a major application of lime in the treatment of wastewater.
Other salts such as iron sulfates Fe2(SO4)3 and FeSO4, chromium sulfate Cr2(SO4)3, and some special polymers are also useful. Other ions such as sodium, chloride, calcium, magnesium, and potassium also affect the coagulation process. So do temperature, pH, and concentration.
Disposal of coagulation sludge is a concern, however.
Sedimentation let the water sit around to let the floculated or coagulated particles to settle out. It works best with relatively dense particles (e.g. silt and minerals), while flotation works better for lighter particles (e.g. algae, color). A settling tank should be big enough so that it takes a long time (ideally 4 hours +) to get through. Inlets and outlets are designed so the water moves slowly in the tank. Long and narrow channels are installed to let the water to snake its way through the tank. The settled particles, sludge, must occasionally be removed from the tanks. The water is next ready to be filtered. Sedimentation is used in pre-treatment and wastewater treatment.
Filtration
Filtration is the process of removing solids from a fluid by passing it through a porous medium. Coarse, medium, and fine porous media have been used depending on the requirement. The filter media are artificial membranes, nets, sand filter, and high technological filter systems. The choice of filters depends on the required filtering speed and the cleanness requirement. The flow required for filtration can be achieved using gravity or pressure. In pressure filtration, one side of the filter medium is at higher pressure than that of the other so that the filter plane has a pressure drop. Some portion of this filter type must be enclosed in a container.
The process of removing the clogged portion of the filter bed by reversing the flow through the bed and washing out the solid is called back washing. During this process, the solid must be removed out of the system, but otherwise the filters must be either replaced or taken out of service to be cleaned.
 Aqua-Rain manufacture water filters as shown here. This unit consists of four filters. Regarding the filtering system, its technical info gave the following statement.
Aqua-Rain manufacture water filters as shown here. This unit consists of four filters. Regarding the filtering system, its technical info gave the following statement.
At the heart of the AquaRain™ Water Filtration System are Marathonr State-of-the-art ceramic elements utilizing a long-proven filtration process that is over 100 years old which will safely remove dangerous waterborne pathogens such as cysts (Cryptospordium, Giardia lamblia) and bacteria (E. coli, Samonelli typhus, etc...). These innovative Marathonr ceramic elements are also filled with a high grade silvered granulated activated carbon (GAC). The GAC reduces pesticides, chemicals, chlorine, tastes & odors, while leaving the naturally occurring minerals found in the water unaffected.
The units are designed for emergency and perhaps undeveloped countries.
AquaSelect of Mississauga has a pitcher water filter system, and its cartridge contains hundreds of high efficiency activated carbon and ion exchange beads, its web site claims. Brita filters is very popular.
Aeration
Bringing air into intimate contact with water for the purpose of exchanging certain components between the two phases is called aeration. Oxygenation is one of the purposes of aeration. Others are removal of volatile organic substances, hydrogen sulfide, ammonia, and volatile organic compounds.
A gas or substance dissolved in water may further react with water. Such a reaction is called hydration. Ionic substance dissolve due to hydration, for example:
\[\ce{HCl (g) + x H2O <=> H(H2O)_{x}^{+} + Cl(aq)^{-}}\]
\[\ce{H2S <=> H^{+}(aq) + HS^{-}(aq)}\]
These reactions are reversible, and aeration may also causes dehydration resulting in releasing the gas from water. Henry's law is applicable to this type of equilibrium for consideration. Methods of aeration are
- Diffused aeration - Air bubbles through water.
- Spray aeration - Water is sprayed through air.
- Multiple-tray aeration - Water flows through several trays to mix with air.
- Cascade aeration - Water flows downwards over many steps in the form of thin water falls.
- Air stripping - A combination of multiple tray and cascade technique plus random packed blocks causing water to mix thoroughly with air.
Reverse Osmosis Water Filter System
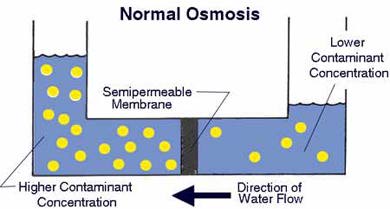 In the following discussion, a dilute solution and a concentrated solution are considered. The dilute solution can be a clean water whereas the concentrated solution contains undesirable solute (electrolyte or others).
In the following discussion, a dilute solution and a concentrated solution are considered. The dilute solution can be a clean water whereas the concentrated solution contains undesirable solute (electrolyte or others).
When a compartment containing a dilute solution is connected to another compartment containing a concentrated solution by a semipermeable membrane, water molecules move from the dilute solution to concentrated solution. This phenomenon is called osmosis. Pig bladders are natural semipermeable membranes. As the water molecules migrate through the semipermeable membrane, water level in the solution will increase until the (osmotic) pressure prevents a net migration of water molecules in one direction. A pressure equivalent to the height difference is called the osmotic pressure. The illustration given on the right is from the PurePro, one of the many companies that manufacture reverse osmosis water filter devices. Millipore also use this technique.
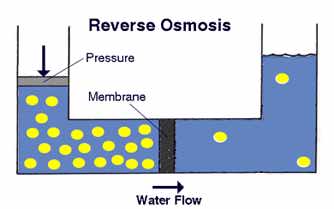 By applying pressure in the higher concentration solution, water molecules migrate from a high concentration solution to a low concentration solution. This method is called reverse osmosis water filter system. The concept of reverse osmosis is illustrated in the diagram here from PurePro.
By applying pressure in the higher concentration solution, water molecules migrate from a high concentration solution to a low concentration solution. This method is called reverse osmosis water filter system. The concept of reverse osmosis is illustrated in the diagram here from PurePro.
In this technique, the membrane must be able to tolerate the high pressure, and prevent solute molecules to pass through. Regarding membranes, PurePro made the following statement:
Semipermeable membranes have come a long way from the natural pig bladders used in the earlier osmosis experiments. Before the 1960's, these membranes were too inefficient, expensive, and unreliable for practical applications outside the laboratory. Modern advances in synthetic materials have generally solved these problems, allowing membranes to become highly efficient at rejecting contaminants, and making them tough enough to withstand the greater pressures necessary for efficient operation.
This technology certainly works, and it has been used to convert salt (ocean or sea) water into fresh water. With this technique, the water with higher concentration is discharged. Thus, this technology is costly in regions where the water cost is high. Free Drinking Water also uses reverse osmosis filter system for domestic applications.




 Charcoal consists of microcrystallites of graphite. The particles are so small in charcoal that they were considered amorphous. The crystal structure of graphite consists of layers of hexagonal networks, stacked on top of each other. Today, making activated carbon is a new and widely varied industry. Other molecules attach themselves to the porous surface and dangling carbons in these microcrystallites.
Charcoal consists of microcrystallites of graphite. The particles are so small in charcoal that they were considered amorphous. The crystal structure of graphite consists of layers of hexagonal networks, stacked on top of each other. Today, making activated carbon is a new and widely varied industry. Other molecules attach themselves to the porous surface and dangling carbons in these microcrystallites.
 In the following discussion, a dilute solution and a concentrated solution are considered. The dilute solution can be a clean water whereas the concentrated solution contains undesirable solute (electrolyte or others).
In the following discussion, a dilute solution and a concentrated solution are considered. The dilute solution can be a clean water whereas the concentrated solution contains undesirable solute (electrolyte or others). By applying pressure in the higher concentration solution, water molecules migrate from a high concentration solution to a low concentration solution. This method is called
By applying pressure in the higher concentration solution, water molecules migrate from a high concentration solution to a low concentration solution. This method is called