8.4: Chemical Contamination of Water
- Page ID
- 204229
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The US Safe Drinking Water Act defines the term "contaminant" as meaning any physical, chemical, biological, or radiological substance or matter in water. Therefore, the "contaminant" definition very broadly applies as being anything other than water molecules. Drinking water may reasonably be expected to contain at least small amounts of some contaminants. Some drinking water contaminants may be harmful if consumed at certain levels in drinking water while others may be harmless. The presence of contaminants does not necessarily indicate that the water poses a health risk.
Air Pollutants
Water can become contaminated at any part of the water cycle. Air pollution can affect water vapor and water liquid. Combustion sources like vehicles and power plants generate compounds like \(\ce{NO_xs}\), \(\ce{SO_xs}\), \(\ce{CO}\), \(\ce{CO2}\), and other various inorganic and organic volatile organic compounds (VOC) species. Some of these compounds can become soluble in water. This could affect pH (or acidity) level of water surface water. Normally, the pH of water is a neutral value (or pH= 7). When \(\ce{NO_xs}\), \(\ce{SO_xs}\), \(\ce{CO}\), \(\ce{CO2}\) enter the water cycle, then the pH level is lowered below 7.0. If these gases are absorbed in rain clouds, then acid rain results. Specific acids involved in acid rain are sulfuric, nitric, and carbonic. This environmental problem affects living organisms and building materials. Acid solutions can corrode metals and make them soluble as well.
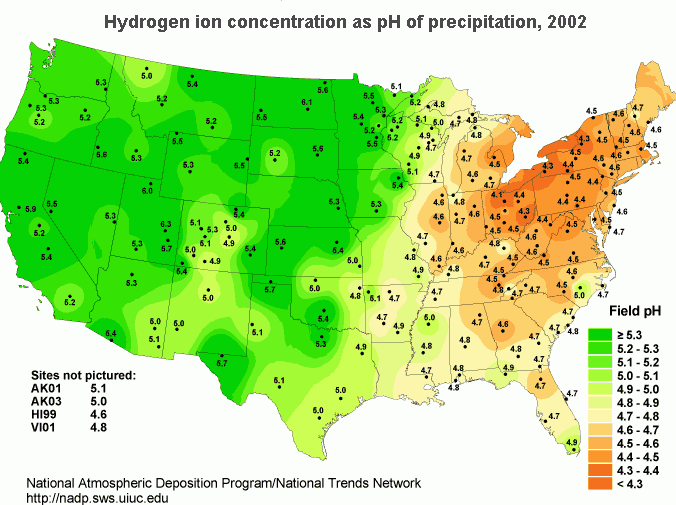
In the United States, the northeast has the most problems with acid rain. Concentrated populations that use electrical energy and vehicles contribute greatly to the pH reduction of rainwater. Reducing gaseous output requires capping combustion sources (vehicles and power plants). Acidity in rain is measured by collecting samples of rain and measuring its pH. To find the distribution of rain acidity, weather conditions are monitored and rain samples are collected at sites all over the country (Figure \(\PageIndex{6}\)). The areas of greatest acidity (lowest pH values) are located in the Northeastern United States. This pattern of high acidity is caused by a large number of cities, the dense population, and the concentration of power and industrial plants in the Northeast. In addition, the prevailing wind direction brings storms and pollution to the Northeast from the Midwest, and dust from the soil and rocks in the Northeastern United States is less likely to neutralize acidity in the rain.
Acid rain from sulfuric acid
However, the majority of acid rain is accounted for by the presence of sulfuric acid (\(\ce{H2SO4}\)).
\[ \ce{SO2(g) + O2(g) -> SO3(g)}\]
Sulfur dioxide reacts with water to form sulfuric acid
\[\ce{SO3(g) + H2O -> H2SO4} \label{eq10}\]
Although sulfuric acid may be produced naturally in small quantities from biological decay and volcanic activity, it is produced almost entirely by human activity, especially the combustion of sulfur-containing fossil fuels in power plants (Figure \(\PageIndex{6}\)).
Acid rain from nitric acid
Some of acid rain is accounted for by nitric acid (\(\ce{HNO3}\)) that originates from natural processes, but especially from high-temperature air combustion, such as occurs in car engines and power plants, that produces large amounts of \(\ce{NO}\) gas. This gas then forms nitric acid via several steps. First nitric oxide (\(\ce{NO}\)) is formed during lightning storms by the reaction of nitrogen and oxygen
\[ \ce{N2 (g) + O2(g) -> 2NO(g)} \label{eq1}\]
and \(\ce{NO}\) is oxidized to nitrogen dioxide (\(\ce{NO2}\))
\[ \ce{NO(g) + 1/2 O2(g) -> NO2 (g)} \label{eq2}\]
\(\ce{NO2(g)}\) then reacts with water droplets to generate nitric acid (\(\ce{HNO3}\))
\[\ce{3NO2(g) + H2O(l) -> 2HNO3(aq) + NO(g)}\]
| Combustion products | Sources |
|---|---|
| CO2 and CO | Combustion of any material (any fuel or tree) |
| NOX (NO2 and NO3) | High-temperature combustion of any fuel ( gas, diesel, or coal), a product of lightning |
| SOx (SO2 and SO3) | Combustion of sulfur-based fuels (diesel and coal), volcanic release |
| VOC (volatile organic compound) | Combustion of any carbon-based fuel (gas, diesel, or coal), fumes from paints or solvents |
Inorganic Contaminants
Solubility of these species can be determined by consulting a solubility table (e.g., Table E3). These chemicals might be naturally present in a water source. Industry and agriculture also use and dispose of these types of chemicals as well. In determining the source of any pollutant, it is helpful to know a region's geology and proximity to chemical consumers. In this section, we will focus on a few inorganic species that can affect drinking water quality.
Hard Water
When water is described as being hard, then this means it has a large concentration of minerals. The ions that contribute to this problem are calcium, magnesium, carbonate, and iron. Hard water is not toxic but can affect industrial processes. These ions will bond together to form insoluble salts that are called precipitates. Building up of these newly formed solids is called scale. Precipitates can collect in boilers, cooling towers, and any equipment that employs water. This buildup can affect how the equipment works by damaging it internally.
Video
Watch this video below to get an idea of what hard water is.
Please be sure to note that the speaker is incorrect when saying that calcium carbonate can be boiled off. Ionic compounds cannot be removed by boiling. The speaker should mention distillation when discussing the separation of calcium carbonate from the water.
Soft Water
Soft water does not contain an excessive amount of minerals. Instead, potassium and sodium ions are present. Soft water does not form scale but does produce excessive lathers with soap. Although industry and consumers prefer soft water, the sodium content can affect health if ingested at a high concentration.
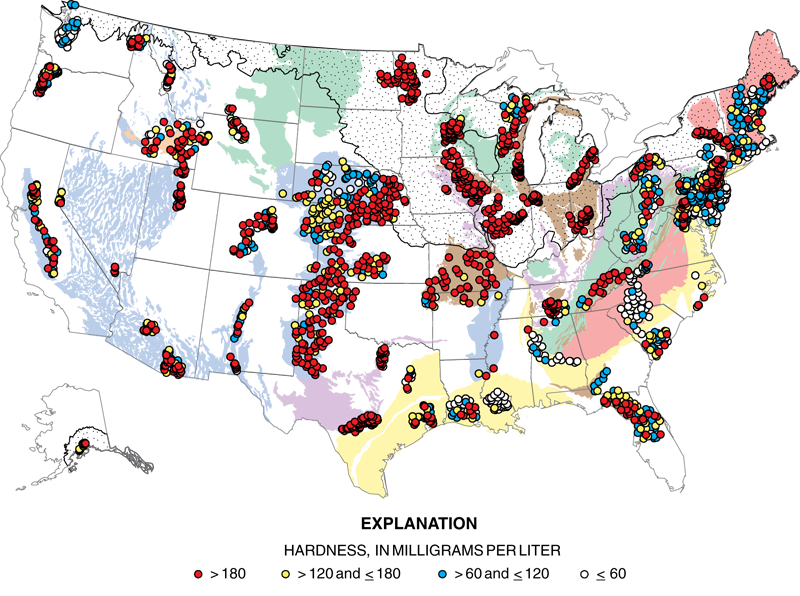
Eutrophication
When pollutants enter a water system they originate from either point or non-point sources. If a single identifiable source can be located, then the pollutant originated from a point source. In contrast, nonpoint sources indirectly contaminant water, air, or soil. Examples of non-point sources would be run-off and acid rain. Obviously, non-point pollutants are more difficult to regulate.
In agriculture, nitrates are used as fertilizers. Farmers can choose to use synthetic or natural (manure) nitrates. Regulating concentrations of these species could be difficult if all sources are not investigated and monitored. An excessive amount of these substances leads to eutrophication. Many nitrate and phosphate containing compounds are soluble in water. Once dissolved, these substances can travel to lakes and ponds. Here, these chemicals activate plants and algae to grow. Overgrowth of plant-life in water can inhibit oxygen from entering the water. As a result, aquatic animals are more likely to die in this environment. In addition, decomposition of organic material (once living) will be slow and smelly.

.jpg?revision=1&size=bestfit&width=503&height=338)
Image taken from: https://theskyguys.ca/2017/02/water-...e-great-lakes/ (I took this pic from a site that I don't think is cc). and Image used with permission (CC BY-SA 2.0; N Chadwick).
Eutrophication can be halted in phosphate contamination is controlled. This involves limiting the use of some fertilizers and removing phosphates from detergents. Reducing nitrates can be a more challenging task. All compounds containing this polyatomic ion are extremely soluble. In addition, controlling nitrate concentration from fecal material can be difficult.
Toxic Metals
Toxic metals, including "heavy metals," are individual metals and metal compounds that negatively affect people's health. Some toxic, semi-metallic elements, including arsenic and selenium, are discussed in this page. In very small amounts, many of these metals are necessary to support life. However, in larger amounts, they become toxic. They may build up in biological systems and become a significant health hazard.
Lead (Action Level is 0.015 ppm)
Occupational exposure to lead is one of the most prevalent overexposures. Industries with high potential exposures include construction work, most smelter operations, radiator repair shops, and firing ranges.
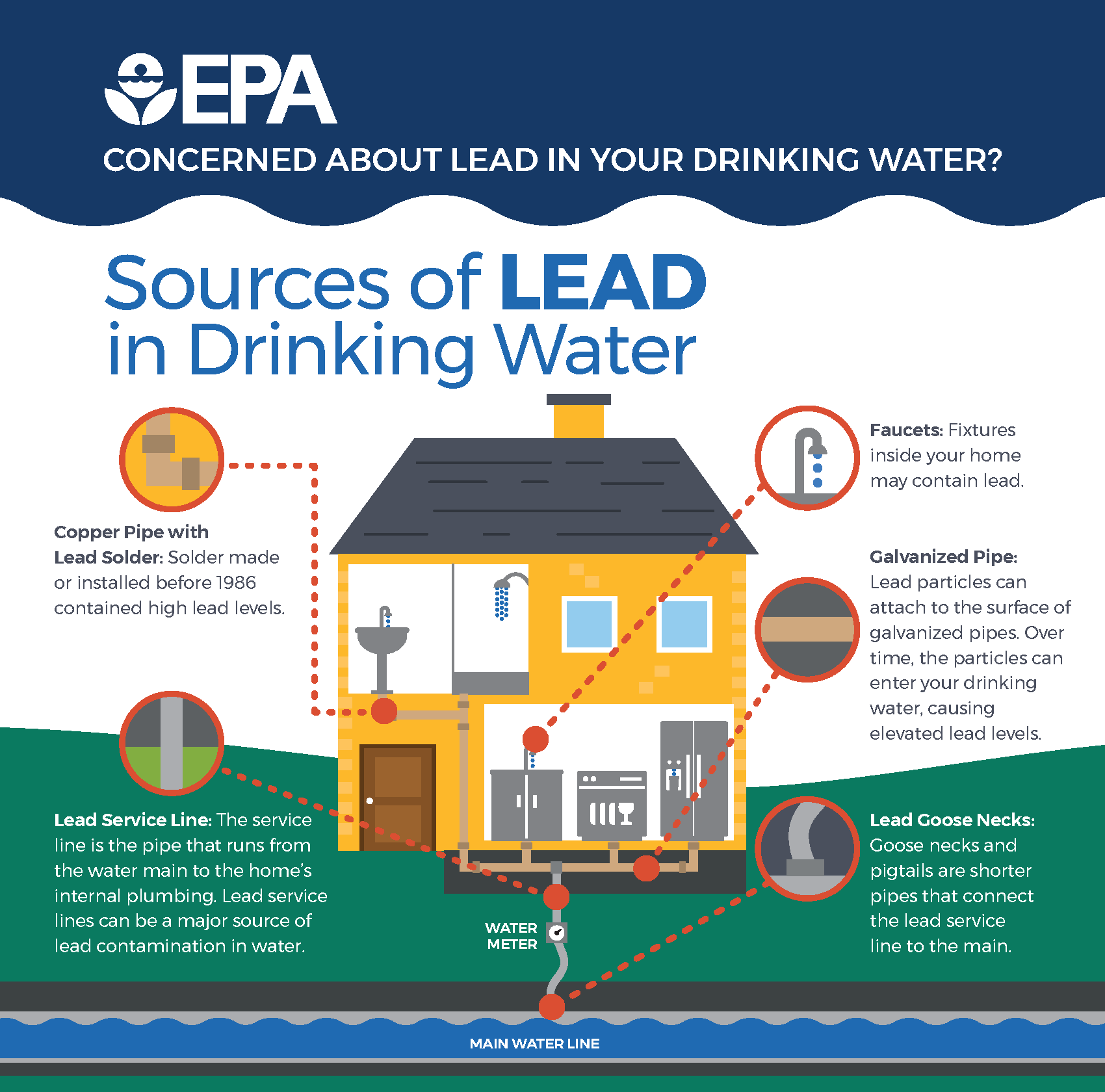

https://media.defense.gov/2017/May/2...-PO640-001.JPG (please add this picture beside the house)
Arsenic (0.010 ppm)
Common sources of exposure to higher-than-average levels of arsenic include near or in hazardous waste sites and areas with high levels naturally occurring in soil, rocks, and water. Exposure to high levels of arsenic can cause death.
Mercury (0.002 ppm)
Common sources of mercury exposure include mining, production, and transportation of mercury, as well as mining and refining of gold and silver ores. High mercury exposure results in permanent nervous system and kidney damage.

Cadmium (0.005 ppm)
Cadmium is an extremely toxic metal commonly found in industrial workplaces, particularly where any ore is being processed or smelted. Several deaths from acute exposure have occurred among welders who have unsuspectingly welded on cadmium-containing alloys or with silver solders.
Uranium (30 ppb)
Deadly Organics
Dioxin ( 3.0 x 10-8 ppm)
Dioxins are mainly byproducts of industrial practices. They are produced through a variety of incineration processes, including improper municipal waste incineration and burning of trash, and can be released into the air during natural processes, such as forest fires and volcanoes. Almost every living creature has been exposed to dioxins.
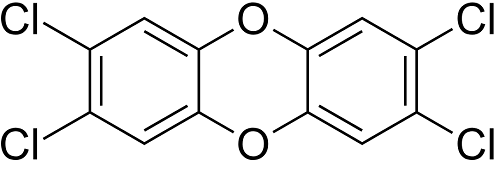
PCB (5.0 x 10-4 ppm)


