6.6: Common "work-up"
- Page ID
- 535988
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Common Washes
Water
The most common wash in separatory funnels is probably water. It is cheap, non-hazardous, and works well to remove may impurities found alongside a desired product. Water can potentially remove water-soluble impurities from an organic layer, as long as they are present in quantities that do not exceed their water solubility. The following are common materials that can be removed with a water wash: unconsumed acid or base, many ionic salts, and compounds that can hydrogen bond with water and are relatively small (e.g. CH3CH2OH or CH3COCH3).
To demonstrate the effectiveness of a water wash, a Fischer esterification reaction was conducted to produce isoamyl acetate. In this reaction, an excess of acetic acid is used to drive the reaction through Le Chatelier's principle, and the acetic acid had to be removed from the product during the purification process.
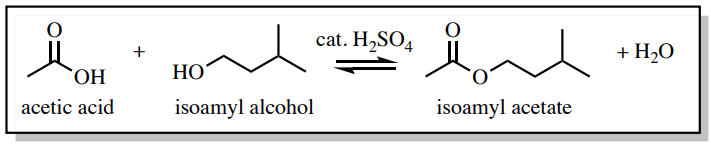
The reaction was then "worked up" by pouring the reaction mixture into a separatory funnel and washing the organic layer with water, sodium bicarbonate, and brine in succession. The main purpose of the water wash was to remove the majority of the catalytic sulfuric acid and the excess acetic acid, while the sodium bicarbonate wash neutralized the rest.
The sodium bicarbonate wash in this example was necessary (and discussed in the next section) because a water wash alone may not fully remove the acetic acid. It's important to know that when a compound is "water soluble" it does not necessarily mean it is "organic insoluble", a common misconception that arises from the "like dissolves like" principle. The ability of acetic acid and other polar compounds to dissolve in the organic layer of a separatory funnel should not be ignored.
Sodium Bicarbonate and Sodium Carbonate
A normal part of many work-ups includes neutralization. It is important to neutralize any organic solvent that was exposed to an acidic or basic solution as trace acid or base may cause undesired reactions to occur when the solutions are concentrated.
Aqueous solutions of saturated sodium bicarbonate (NaHCO3) and sodium carbonate (Na2CO3) are basic, and the purpose of these washes is to neutralize an organic layer that may contain trace acidic components. Even if an organic layer should not in theory dissolve very polar components such as acid, acid sometimes "hitches a ride" on polar components that may dissolve in an organic layer, such as small amounts of alcohol or water.
Neutralizing with sodium bicarbonate and sodium carbonate solutions produces carbonic acid (H2CO3), which is in equilibrium with water and carbon dioxide gas. This means that solutions often bubble during a neutralization wash in a separatory funnel.
Safety note: To prevent excess pressure from being generated by the release of carbon dioxide gas into a separatory funnel during neutralization, the layers should be gently swirled together before placement of the stopper. They should be vented directly after inversion, and more frequently than usual.
Testing the pH After a Wash
To test whether a base wash with NaHCO3 or Na2CO3 was effective at removing all the acid from an organic layer, it is helpful to test the pH. It is not possible to test the pH of an organic solution directly, however it is possible to test the pH of an aqueous solution that the organic solution has been in contact with using pH paper.
Brine (Saturated NaCl)
In some experiments, an organic layer may be washed with brine, which is a saturated solution of NaCl. The purpose of this wash is to remove large amounts of water that may be dissolved in the organic layer. Brine works to remove water from an organic layer because it is highly concentrated (since NaCl is so highly water soluble). A saturated NaCl solution causes water to draw into the solution from the organic layer.
Drying Agents
An organic layer is always treated with a drying agent after having been exposed to water in a separatory funnel. Drying agents are anhydrous inorganic materials that favorably form "hydrates", which incorporate water molecules into their solid lattice structure (for example, Na2SO4⋅7H2O). A drying agent is swirled with an organic solution to remove trace amounts of water. If drying agents are used to remove water, you might wonder "Why bother with brine; why not use lots of drying agent when the time comes?" The main reason to limit the amount of water present in an organic solution before the drying agent step is that the drying agent will often adsorb the compound along with water. Using as little as possible will maximize the yield.

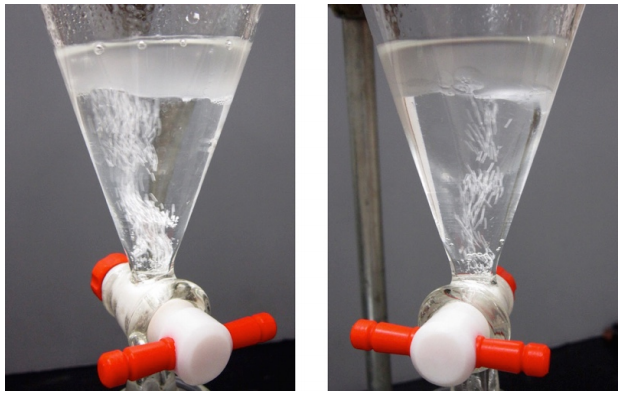
The most useful drying agents indicate when they have completely absorbed all of the water from the solution. Anhydrous magnesium sulfate (MgSO4) is a fine, loose powder, but its hydrate is clumpy and often clings to the glass. A typical drying procedure is to add anhydrous MgSO4 to an organic solution until it stops clumping and fine particles are seen, which indicate that there is no longer water available to form the clumpy hydrates.
Anhydrous calcium sulfate (CaSO4), can be purchased containing a cobalt compound that is blue when dry and pink when wet (this is then sold under the name Drierite). In this way, blue Drierite can be used as a visual indicator for the presence of water.

In some procedures Na2SO4 or CaCl2 are used if the solution is incompatible with MgSO4. An advantage to these drying agents is that their granules are not easily dispersed, allowing for the solutions to be easily decanted. In many situations drying agents are interchangeable.
| Drying Agent | Hydrate formula(s) | Practical Comments | Other Comments |
|---|---|---|---|
| Magnesium sulfate | MgSO4⋅7H2O | Quickly removes most water, and can hold a lot for its mass (0.15-0.75g water per g desiccant). Is a fine powder, so must be gravity filtered. Its high surface area means it will somewhat adsorb compound: be sure to rinse after filtering. | Mg(H2O)2+4 is somewhat acidic, so is incompatible with highly acid-sensitive groups. |
| Sodium Sulfate |
Na2SO4⋅7H2O Na2SO4⋅10H2O |
Removes water at a moderate rate, so the solution should be allowed to sit with the drying agent for some time. Can hold a lot of water for its mass (1.25g water per g desiccant), but may leave small amounts of water remaining. Solutions with Na2SO4 can usually be decanted. | Cannot dry diethyl ether well unless a brine wash was used. |
| Calcium chloride |
CaCl2⋅2H2O CaCl2⋅6H2O |
Quickly removes water well, although larger quantities are needed than other drying agents (holds 0.30g water per g desiccant). If using a fine powder, the solution must be gravity filtered and drying agent rinsed. If using pellets, the solution should be allowed to sit for a few minutes, then decanted. | Absorbs water as well as methanol and ethanol. |
| Calcium sulfate (Drierite) |
CaSO4⋅12H2O CaSO4⋅2H2O |
Quickly removes water, but needs large quantities as it holds little water per gram. Are most often used in desiccators and drying tubes, not with solutions. |
Drying Agents Procedure
- The organic solution to be dried must be in an Erlenmeyer flask, as solutions can easily splash out of beakers when swirled.
- Add a small portion of drying agent to the flask, the size of one pea and swirl the solution. Be sure to close the jar of drying agent when not in use, as the reagents are hygroscopic. After a short period of time, inspect the mixture closely.
- If the entire drying agent clumps into pieces that are much larger than the original size, there is still water remaining in the flask. Add another portion of drying agent and swirl.
- A solution is nearing dryness when fine particles are noticed that don't cling to other particles or to the glass when swirled. A wet organic solution can be cloudy, and a dry one is always clear.
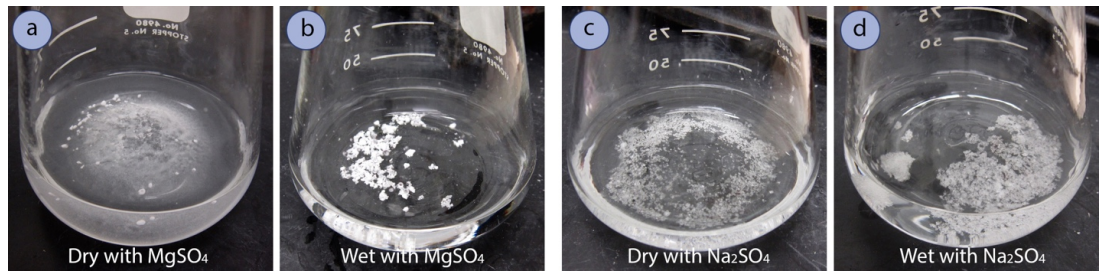
- When the solution is dry, separate the drying agent from the solution:
- Carefully decant the solution into an appropriately sized round-bottomed flask, being sure to fill the flask no more than halfway.
- If using MgSO4, instead of decanting, gravity filter the solution into an appropriately sized round-bottomed flask.
- With all drying agents, rinse the drying agent with a few mL of fresh organic solvent, and add the rinsing to the round-bottomed flask.
Adapted from Common Extraction Washes by Lisa Nichols.

