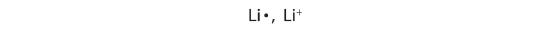3.3: Ions
- Page ID
- 393865
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Distinguish the difference between the two types of ions.
- Describe ion formation using electron configurations.
- Predict ionic charge.
Ions
As introduced in Chapter 2, atoms contain a nucleus with neutrons and positively charged protons, surrounded by negatively charged electrons. In an atom, the total number of electrons, negative charge, equals the total number of protons, positive charge, and therefore, atoms are electrically neutral or uncharged. If an atom loses or gains electrons, it will become a positively or negatively charged particle, called an ion. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. For example, a sodium atom with one less electron is a cation, Na+, with a +1 charge (Figure \(\PageIndex{1}\)).

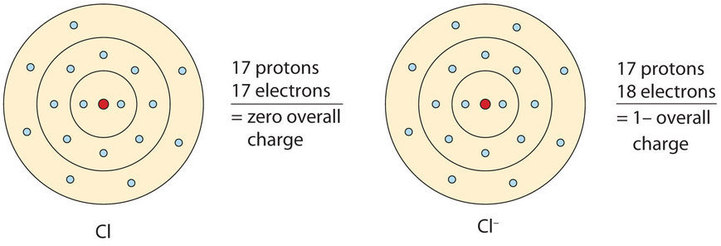
When an atom gains one or more electrons, it becomes a negatively charged anion, because there are more electrons than protons. When chlorine gains one electron it forms a chloride ion, Cl–, with a –1 charge (Figures \(\PageIndex{2}\))
The names for positive and negative ions are pronounced CAT-eye-ons (cations) and ANN-eye-ons (anions), respectively.
Predicting Ionic Charge
Ions are formed when an atom loses or gains electrons. These electrons are usually lost from and gained into the valence shell, or outermost energy level. Therefore, it is useful to look at electron configurations to further illustrate ion formation and electron transfer between atoms.
The electron configuration for sodium shows that there are ten core electrons and one valence electron in the third energy level. When sodium loses the single valence electron, forming the cation Na+, the electron configuration is now identical to that of neon, a stable noble gas.
\[\begin{array}{lcl} \ce{Na} & \rightarrow & \ce{Na^+} + \ce{e^-} \\ 1s^2 \: 2s^2 \: 2p^6 \: 3s^1 & & 1s^2 \: 2s^2 \: 2p^6 \end{array}\]
Chlorine also has ten core electrons and valence electrons in the third energy level. However, chlorine has seven valence electrons, one less than the noble gas argon, which has eight valence electrons. Thus, chlorine will gain one electron, forming the anion, Cl–, and achieving a stable noble gas configuration.
\[\begin{array}{lcl} \ce{Cl} + \ce{e^-} & \rightarrow & \ce{Cl^-} \\ 1s^2 \: 2s^2 \:2p^6 \: 3s^2 \: 3p^5 & & 1s^2 \: 2s^2 \: 2p^6 \: 3s^2 \: 3p^6 \end{array}\]
Cations are named using the element name plus "ion" to indicate it is charged. Anions are named by changing the element name ending to "ide". For example, a magnesium ion is formed when neutral magnesium loses electrons and a fluoride ion is formed when neutral fluorine gains electrons.
When atoms form ions, they tend to reach a full valence shell by gaining enough electrons to have eight electrons in the valence shell or losing the electrons in their original valence shell; the lower shell, now the valence shell, has eight electrons in it. For whatever reason, having eight electrons in a valence shell is a particularly energetically stable arrangement of electrons. The trend that atoms like to have eight electrons in their valence shell is called the octet rule. When atoms form compounds, the octet rule is not always satisfied for all atoms at all times, but it is a very good rule of thumb for understanding the kinds of bonding arrangements that atoms can make
Example \(\PageIndex{1}\)
Write the electron configuration of aluminum atom (Z = 13) and underline the valence electrons. How many electrons are gained/lost to form an aluminum ion, Al3+? Write the electron configuration for this ion.
Solution
The electron configuration of Al atom is 1s22s22p63s23p1. Aluminum has three valence electrons in the third energy level, (3s23p1). The cation, Al3+, is formed when these three valence electrons are lost, leaving the configuration for the noble gas neon, 1s22s22p6.
Exercise \(\PageIndex{1}\)
Write the electron configuration of oxygen atom (Z = 8) and underline the valence electrons. How many electrons are gained/lost to form an oxide ion, O2–? Write the electron configuration for this ion.
- Answer
-
The electron configuration of O atom is 1s22s22p4. Oxygen has six valence electrons in the second energy level, (2s22p4). The anion O2− is formed when two electrons are gained in the valence shell. The resulting electron configuration, 1s22s22p6, is also identical to the configuration for the noble gas neon.
Key Takeaways
- Ions can be positively charged or negatively charged.
- Ionic charge relates to valence electrons and valence shells.
Contributors
- Lisa Sharpe Elles, University of Kansas
Exercises
-
What are the two types of ions?
-
When the following atoms become ions, what charges do they acquire?
- Li
- S
- Ca
- F
4. Identify each as a cation, an anion, or neither.
- H+
- Cl−
- O2
- Ba2+
- CH4
- CS2
5. Identify each as a cation, an anion, or neither.
- NH3
- Br−
- H−
- Hg2+
- CCl4
- SO3
6. Write the electron configuration for each ion.
- Li+
- Mg2+
- F−
- S2−
7. Write the electron configuration for each ion.
- Na+
- Be2+
- Cl−
- O2−
Answers
-
Cations (positive charged) and anions (negative charged)
-
- 1+
- 2−
- 2+
- 1−
4.
- cation
- anion
- neither
- cation
- neither
- neither
- neither
- anion
- anion
- cation
- neither
- neither
6.
- 1s2
- 1s22s22p6
- 1s22s22p6
- 1s22s22p63s23p6
- 1s22s22p6
- 1s2
- 1s22s22p63s23p6
- 1s22s22p6
b. 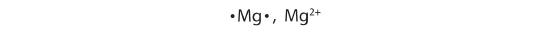
c. 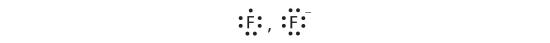
d. 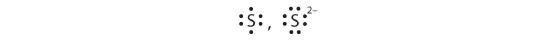
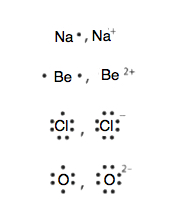
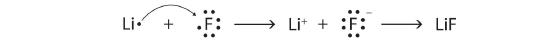
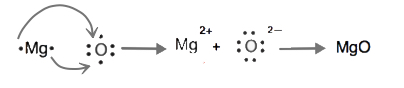
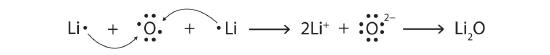
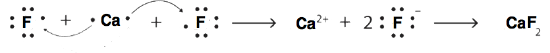
14. 1+
16. 2−
17. 1−