2.2.4.6F: Troubleshooting
- Last updated
- Save as PDF
- Page ID
- 406433
Crystallization is Too Quick
Rapid crystallization is discouraged because impurities tend to become incorporated into the crystal, defeating the purpose of this purification technique. It may be acceptable for crystallization to start immediately after removing the flask from the heat source, but if a large amount of solid is formed then the compound is crystallizing too fast.
An ideal crystallization has some crystals forming in approximately 5 minutes, and growth continuing over a period of 20 minutes. Below are methods that can be used to slow the growth of crystals:
- Place the solid back on the heat source and add extra solvent (perhaps \(1\)-\(2 \: \text{mL}\) for \(100 \: \text{mg}\) of solid), so that you have exceeded the minimum amount of hot solvent needed to dissolve the solid. Although more compound will dissolve in the mother liquor, the compound will stay soluble longer once set aside to cool.
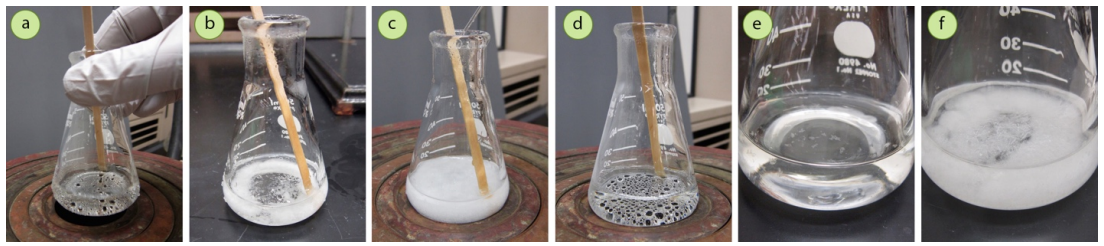
For example, in the crystallization of trans-cinnamic acid with a mixed solvent of methanol and water, use of the minimum amount of hot solvent to dissolve the solid (Figure 3.60a) resulted in the solid immediately crashing out of solution when the solution was taken off the heat source (Figure 3.60b). To remedy this problem, the solid was placed back on the steam bath, additional methanol (soluble solvent) was added, brought to a boil to dissolve the solid, and then cooled. The solid then began to crystallize much more slowly (Figure 3.60c), taking a period of 15 minutes to fully crystallize.
- If the minimal amount of hot solvent needed to dissolve the solid reached a height of less than \(1 \: \text{cm}\) in the flask, the flask may be too big for the crystallization. A shallow solvent pool has a high surface area, which leads to fast cooling. Transfer the solution to a smaller flask (using some solvent to rinse the flask, and then boil away the same amount of solvent used for rinsing) and repeat the crystallization.
- Be sure to use a watch glass over the top of the Erlenmeyer flask to trap heat, and set the flask atop some material to insulate the bottom (several paper towels, a wood block, or cork ring). An inverted beaker could also be tried to create an insulating atmosphere around the cooling flask.
Crystallization Doesn't Happen
It can be quite frustrating to set aside the dissolved solution to cool and have no crystals form at all. Methods to initiate crystallization were discussed in great detail previously. To summarize, here are the methods that can be tried (in hierarchical order) to form crystals depending on the appearance of the solution:
- If the solution is cloudy, scratch the flask with a glass stirring rod.
- If the solution is clear,
- First try scratching the flask with a glass stirring rod.
- Add a seed crystal (a small speck of crude solid saved from before the crystallization was begun, or a bit of pure solid from the reagent jar).
- Dip a glass stirring rod into the solution, remove it, and allow the solvent to evaporate to produce a thin residue of crystals on the rod (Figure 3.61). Then touch the rod to the solution's surface, or stir the solution with the rod to dislodge small seed crystals.
- Return the solution to the heat source an boil off a portion of solvent (perhaps half), then cool again.
- Lower the temperature of the cooling bath.
- If very few crystals are seen, there is likely too much solvent. Return the solution to the heat source and boil off a portion of solvent, then cool again.
- If all else fails, the solvent can always be removed by rotary evaporation to recover the crude solid. Another crystallization can be attempted, perhaps with a different solvent system.

The Yield is Poor
A crystallization may result with a really poor yield (e.g. less than \(20\%\)). There are several reasons why this might happen:
- Too much solvent may have been used during the crystallization, and therefore large quantities of compound were lost to the mother liquor. If the mother liquor (the filtrate after suction filtration) has not been disposed of, this can be tested by dipping a glass stirring rod into the mother liquor and letting it dry. If the solvent evaporates to leave a large residue on the rod, there is a lot of compound left in solution. Additional compound may be recovered by boiling away some of the solvent and repeating the crystallization (this is called "second crop crystallization"), or by removing all of the solvent by rotary evaporation and repeating the crystallization with a different solvent.
- Too much solvent may have been used while attempting to dissolve semi-insoluble impurities. If this may have been the case, a hot filtration could have been attempted to remove the impurities. (The solid would have to be recovered from the mother liquor first through rotary evaporation in order to attempt the crystallization again.)
- Too much charcoal may have been used to decolorize the solution (a pitch black solution has too much charcoal). Too much charcoal decreases the yield as charcoal can adsorb the desired compound along with impurities. There is no way to recover the product once it is adsorbed by charcoal.
- If a hot filtration step was used, compound may have been lost in the filter paper and/or on the stem of the funnel. Additionally, too much solvent may have been used when adding a portion to get the system hot before filtration. The extra solvent before filtration adds to the "minimum amount of hot solvent" and if in substantial excess, can cause a loss of compound to the mother liquor.
- If the impure solid was the product of a chemical reaction, the reaction may not have worked well. This could be assessed if a crude mass had been obtained: if the crude mass was very low to begin with, then the low crystallized yield was due to problems with the reaction, not the crystallization.
If a crude mass was not obtained, assessment of the rough volumes of solid before and after crystallization can be used. If the volumes were similar, the loss of yield may not be due to problems with the crystallization. Note that crystallized solids tend to be a lot "fluffier" than crude solids (Figure 3.62), so "eyeballing" solid volumes is only a very rough assessment of quantity.

Liquid Droplets Form (The Solid "Oils Out")
When cooling, a compound may come out of solution as a liquid rather than a solid (Figure 3.63). This process is called "oiling out" and happens when the melting point of the solid is lower than the solution's temperature. This is a problem in crystallization because when compounds liquefy first, they rarely form pure crystals. This is due to the fact that impurities often dissolve better in the liquid droplets than they do in the solvent. Figure 3.63c shows a sample of crude acetanilide that has oiled out (the droplets are impure liquid acetanilide), and the sample is contaminated with a methyl red impurity (which appears red in the low pH of the solution, an artifact of how the crude solid was synthesized). The oily acetanilide droplets appear more colored than the solution, indicating a higher quantity of dissolved methyl red impurity. If an oiled out liquid eventually solidifies, it often forms an impure glass-like non-crystalline solid.
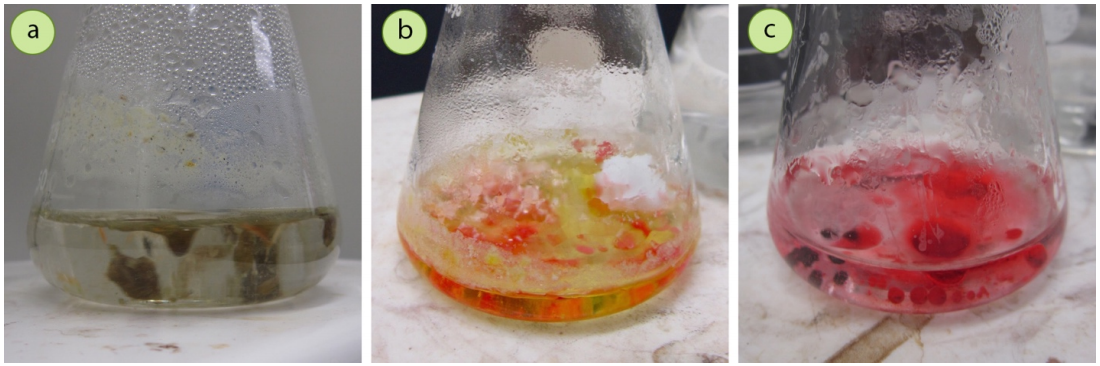
The reasons for oiling out are several, and it can happen while dissolving the solid and during crystallization. It may be that the melting point of the solid is naturally low. It may also be that a solid is so impure that its melting point is dramatically lowered (as impurities lower the melting point).
There are several ways to attempt to fix an oiled out solution:
- Return the sample to the heat source and add a bit more solvent, then cool the solution again. (If using a mixed solvent system, add more of the "soluble solvent"). The solid may have been coming out of solution too quickly (and thus at a temperature above its melting point), so it may stay soluble longer if there is more solvent.
- Add a charcoal step if it was not already a part of the crystallization. The solid may be melting because there are large quantities of impurities, which charcoal can remove. This especially might work if a colored tint is noticed in the hot solution.
If either of these methods fail, recover the crude solid by rotary evaporation and attempt another crystallization. If the failed attempt used a mixed solvent, try a single solvent if possible. Or choose another solvent with similar solubility properties, but with a lower boiling point. For example, if ethanol were used as the solvent the first time, repeat the crystallization using methanol. Methanol has similar solubility properties as ethanol, but its lower boiling point may allow for the solid to come out of solution above its melting point.


