7.6: Writing Lewis Structures for Covalent Compounds
- Page ID
- 367812
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Draw Lewis structures for covalent compounds.
We have established that molecules form by sharing electrons in covalent bonds. Also, that there are several ways in which these electrons could be shared. However, there is a little bit involved in determining how electrons are shared in a particular molecule. We will go through a series of steps in this section which will help determine the correct sharing of electrons in most molecules. Prior to starting on this section, it is important that you know how to determine the number of valence electrons of any element. Please review the previous section where this was discussed prior to starting this section.
Additional ideas related to Lewis structures for molecules will be discussed in the 2 subsections. We will discuss additional considerations for some molecules when more than one Lewis structure might be possible. We will also mention some exceptions to the octet rule.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions.
1. Determine the total number of valence electrons in the molecule or ion.
- Add together the valence electrons from each atom. (Recall that the number of valence electrons is indicated by the position of the element in the periodic table.)
For OF2, for example, the oxygen has 6 electrons and each of the 2 fluorines has 7 electrons for a total of 20 electrons.
- If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to give the total charge on the ion.
For CO32−, for example, we add two electrons to the total because of the −2 charge.
2. Arrange the atoms to show specific connections.
- To begin with, we will consider molecules that have only one central atom along with 2 or more terminal atoms. When we say an atom is central in the molecule, that means each of the other atoms is connected to that central atom. When we say an atom is terminal in the molecule, that means the atom is connected only to the central atom and not to other atoms. (If the molecule has only 2 atoms in it, the distinction between central and terminal doesn’t mean anything.)
- When there is a central atom, chemists usually list this central atom first in the chemical formula (as in CCl4 and CO32−, which both have C as the central atom).
- Hydrogen is always connected to only one other atom, so it is always terminal rather than central even if it is first in the formula.
3. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond.
- In H2O, for example, there is a bonding pair of electrons between oxygen and each hydrogen.
4. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen).
- These added electrons will usually be lone pairs.
- Remember to count the bonding electrons on these terminal atoms while determining if they have enough electrons to satisfy the octet rule.
5. If any electrons are left over, place them on the central atom.
- Determine the number of left over electrons by counting all of the electrons that have already been added (either as lone pairs or in each of the single bonds) and comparing it to the total number of electrons that you counted in the first step. If they are the same, then there are no leftover electrons. If you had more electrons in the first step, then you are able to add the difference between that amount and the amount you have here.
- We will explain later that some atoms are able to accommodate more than eight electrons.
6. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the central atom to achieve an octet.
- This will not change the number of electrons on the terminal atoms, as you are moving the electrons when you do this.
7. Final check
- Always make sure all valence electrons are accounted for. This means that the number of electrons you counted in step one is the same as the number of electrons you can count in step six.
- Each atom has an octet of electrons, except for hydrogen (with two electrons).
Now let’s apply this procedure to some particular compounds, beginning with one we have already discussed.
Write the Lewis Structure for H2O.
Solution
|
Steps for Writing Lewis Structures |
Example \(\PageIndex{1}\) |
|---|---|
| 1. Determine the total number of valence electrons in the molecule or ion. |
Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. |
| 2. Arrange the atoms to show specific connections. |
H O HBecause H atoms are always terminal, the arrangement within the molecule must be HOH. |
|
3. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. 4. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). |
Placing one bonding pair of electrons between the O atom and each H atom gives H -O- Hwith 4 electrons left over. Each H atom has a full valence shell of 2 electrons. |
| 5. If any electrons are left over, place them on the central atom. |
Adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure:
|
| 6. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the central atom to achieve an octet. | Not necessary. |
| 7. Final check. | The Lewis structure gives oxygen an octet and each hydrogen 2 electrons. |
Write the Lewis structure for the \(CH_2O\) molecule
Solution
|
Steps for Writing Lewis Structures |
Example \(\PageIndex{2}\) |
|---|---|
| 1. Determine the total number of valence electrons in the molecule or ion. |
Each hydrogen atom (group 1) has 1 valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. |
| 2. Arrange the atoms to show specific connections. |
Because carbon is first in the formula, we make it the central atom. We are assuming each of the other atoms is a terminal atom connected to the central carbon. |
|
3. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. |
Placing a bonding pair of electrons between each pair of bonded atoms gives the following: 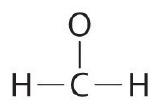 6 electrons are used, and 6 are left over. |
| 4. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). |
Adding all 6 remaining electrons to oxygen (as three lone pairs) gives the following: 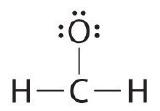 Although oxygen now has an octet and each hydrogen has 2 electrons, carbon has only 6 electrons. |
| 5. If any electrons are left over, place them on the central atom. |
Not necessary. There are no electrons left to place on the central atom. |
| 6. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the central atom to achieve an octet. |
To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond: |
| 7. Final check |
The total number of electrons in the final structure is 12, which matches the total number of electrons we counted in step one. Both the oxygen and the carbon now have an octet of electrons, the O has two bonding pairs and two lone pairs, and C has four bonding pairs. Because both of these requirements have been met, this is an acceptable Lewis electron structure. |
Write Lewis electron structures for CO2 and SCl2, a vile-smelling, unstable red liquid that is used in the manufacture of rubber.
- Answer CO2
-
.


- Answer SCl2
-
.
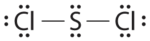
Writing Lewis Structures for Polyatomic Ions
Recall that a polyatomic ion is a group of atoms that are covalently bonded together and which carry an overall electrical charge. The ammonium ion, \(\ce{NH_4^+}\), is formed when a hydrogen ion \(\left( \ce{H^+} \right)\) attaches to the lone pair of an ammonia \(\left( \ce{NH_3} \right)\) molecule in a coordinate covalent bond.
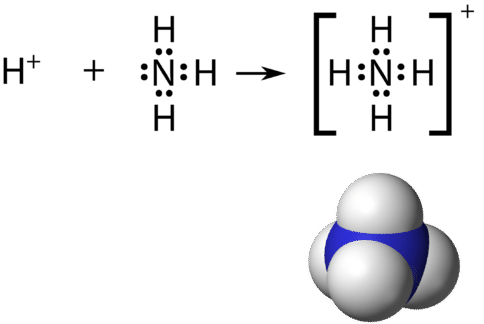
When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion:
\(1 \: \ce{N}\) atom \(= 5\) valence electrons
\(4 \: \ce{H}\) atoms \(= 4 \times 1 = 4\) valence electrons
subtract 1 electron for the \(1+\) charge of the ion
total of 8 valence electrons in the ion
It is customary to put the Lewis structure of a polyatomic ion into a large set of brackets, with the charge of the ion as a superscript outside of the brackets.
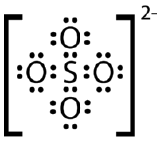
Draw the Lewis electron dot structure for the sulfate ion.
- Answer
Summary
Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed formulas. In Lewis electron structures, we encounter bonding pairs, which are shared by two atoms, and lone pairs, which are not shared between atoms. A process for creating the correct Lewis structures involves 2 criteria: (1) making sure the total number of valence electrons is the same in the molecule as in the sum of the individual atoms which make it up, and (2) making sure that each atom in the structure follows the octet rule. Lewis structures for polyatomic ions follow the same rules as those for other covalent compounds.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Modified by Joshua Halpern (Howard University)
Henry Agnew (UC Davis)