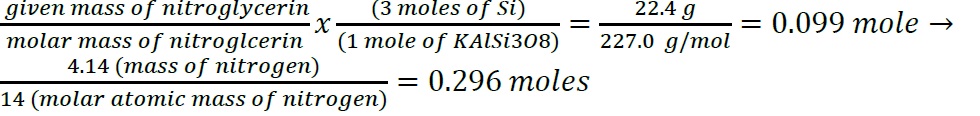4.E: The Mole Concept (Exercises)
- Page ID
- 367776
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The following questions are related to the material covered in this chapter, however they may not be presented in the same order that they were in your chapter. For additional examples also check here (5.2 only) or here (5.75, 5.80, 5.105, 5.106 only)
4.1-4.5: Avogadro's Number, Molar Mass and the Mole
Conceptual Problems
Please be sure you are familiar with the topics discussed in Essential Skills 2 before proceeding to the Conceptual Problems.
- Describe the relationship between an atomic mass unit and a gram.
- Is it correct to say that ethanol has a formula mass of 46? Why or why not?
- If 2 mol of sodium reacts completely with 1 mol of chlorine to produce sodium chloride, does this mean that 2 g of sodium reacts completely with 1 g of chlorine to give the same product? Explain your answer.
- Construct a flowchart to show how you would calculate the number of moles of silicon in a 37.0 g sample of orthoclase (KAlSi3O8), a mineral used in the manufacture of porcelain.
- Construct a flowchart to show how you would calculate the number of moles of nitrogen in a 22.4 g sample of nitroglycerin that contains 18.5% nitrogen by mass.
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 2 before proceeding to the Numerical Problems.
1. Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular mass.
2. Calculate the molecular mass or formula mass of each compound.
- KCl (potassium chloride)
- NaCN (sodium cyanide)
- H2S (hydrogen sulfide)
- NaN3 (sodium azide)
- H2CO3 (carbonic acid)
- K2O (potassium oxide)
- Al(NO3)3 (aluminum nitrate)
- Cu(ClO4)2 [copper(II) perchlorate]
3. Calculate the molecular mass or formula mass of each compound.
- V2O4 (vanadium(IV) oxide)
- CaSiO3 (calcium silicate)
- BiOCl (bismuth oxychloride)
- CH3COOH (acetic acid)
- Ag2SO4 (silver sulfate)
- Na2CO3 (sodium carbonate)
- (CH3)2CHOH (isopropyl alcohol)
8. Calculate the number of moles in 5.00 × 102 g of each substance. How many molecules or formula units are present in each sample?
a. CaO (lime)
b. CaCO3(chalk)
c. C12H22O11 [sucrose (cane sugar)]
d. NaOCl (bleach)
e. CO2 (dry ice)
9. Calculate the mass in grams of each sample.
a. 0.520 mol of N2O4
b. 1.63 mol of C6H4Br2
c. 4.62 mol of (NH4)2SO3
10. Give the number of molecules or formula units in each sample.
a. 1.30 × 10−2 mol of SCl2
b. 1.03 mol of N2O5
c. 0.265 mol of Ag2Cr2O7
11. Give the number of moles in each sample.
a. 9.58 × 1026 molecules of Cl2
b. 3.62 × 1027 formula units of KCl
c. 6.94 × 1028 formula units of Fe(OH)2
12. Solutions of iodine are used as antiseptics and disinfectants. How many iodine atoms correspond to 11.0 g of molecular iodine (I2)?
13. What is the total number of atoms in each sample?
a. 0.431 mol of Li
b. 2.783 mol of methanol (CH3OH)
c. 0.0361 mol of CoCO3
d. 1.002 mol of SeBr2O
14. What is the total number of atoms in each sample?
a. 0.980 mol of Na
b. 2.35 mol of O2
c. 1.83 mol of Ag2S
d. 1.23 mol of propane (C3H8)
15. What is the total number of atoms in each sample?
a. 2.48 g of HBr
b. 4.77 g of CS2
c. 1.89 g of NaOH
d. 1.46 g of SrC2O4
16. Decide whether each statement is true or false and explain your reasoning.
- There are more molecules in 0.5 mol of Cl2than in 0.5 mol of H2.
- One mole of H2 has 6.022 × 1023 hydrogen atoms.
- The molecular mass of H2O is 18.0 amu.
- The formula mass of benzene is 78 amu.
17. Complete the following table.
| Substance | Mass (g) | Number of Moles | Number of Molecules or Formula Units | Number of Atoms or Ions |
|---|---|---|---|---|
| MgCl2 | 37.62 | a. | b. | c. |
| AgNO3 | d. | 2.84 | e. | f. |
| BH4Cl | g. | h. | 8.93 × 1025 | i. |
| K2S | j | k. | l. | 7.69 × 1026 |
| H2SO4 | m. | 1.29 | n. | o. |
| C6H14 | 11.84 | p. | q. | r. |
| HClO3 | s. | t. | 2.45 × 1026 | u. |
18. Give the formula mass or the molecular mass of each substance.
- \(PbClF\)
- \(Cu_2P_2O_7\)
- \(BiONO_3\)
- \(Tl_2SeO_4\)
19. Give the formula mass or the molecular mass of each substance.
- \(MoCl_5\)
- \(B_2O_3\)
- \(UO_2CO_3\)
- \(NH_4UO_2AsO_4\)
Conceptual Answers
- While both are units of mass, a gram is Avogadro’s number of atomic mass units so you would multiply the number of amu by 6.022x10^23 to find total number of grams
- The correct way to state formula mass of ethanol is to show the units of mass which is amu.
- No because moles and weight operate on different set of standards meaning that they’re not equal to each other. This means that moles of different compounds contain different weights. For example, 2 moles of Na = 2 x 22.989 g = 45.98g while 1 mole of Cl = 1 x 35.453 g = 35.453 g Cl. This makes the sodium react completely with chlorine. 2g of sodium would react with = (35.453/45.978) x 2 = 1.542 g Cl
- Construct a flowchart to show how you would calculate the number of moles of silicon in a 37.0 g sample of orthoclase (KAlSi3O8), a mineral used in the manufacture of porcelain
- .
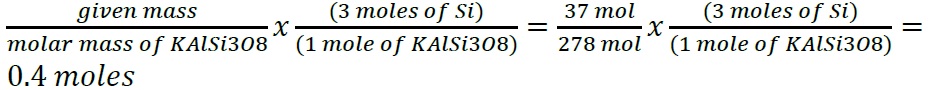
- .
- Construct a flowchart to show how you would calculate the number of moles of nitrogen in a 22.4 g sample of nitroglycerin that contains 18.5% nitrogen by mass.
- The information required to determine the mass of the solute would be the molarity of the solution because once that is achieved, volume of the solution and molar mass of the solute can be used to calculate the total mass. A derivatization that achieves this goes as: Molarity = moles of solute / volume of solution in liter -> Moles = molarity x volume in liter -> Mass= moles x molar mass.
Numerical Answers
1. Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular mass.
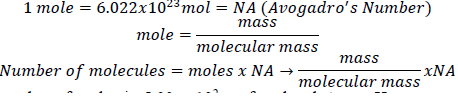
2. Calculate the molecular mass or formula mass of each compound.
- 74.55 amu
- 49.01 amu
- 34.08 amu
- 65.01 amu
- 62.02 amu
- 94.20 amu
- 213.00 amu
- 262.45 amu
3. Calculate the molecular mass or formula mass of each compound.
- 165.88 amu
- 116.16 amu
- 260.43 amu
- 60.05 amu
- 311.80 amu
- 105.99 amu
- 60.10 amu
4. Calculate the molar mass of each compound.
a. 153.82 g/mol
b. 80.06 g/mol
c. 92.01 g/mol
d. 70.13 g/mol
e. 74.12 g/mol
5. Calculate the molar mass of each compound.
a. 92.45 g/mol
b. 135.04 g/mol
c. 44.01 g/mol
d. 40.06 g/mol
6. For each compound, write the condensed formula, name the compound, and give its molar mass.
a. C5H10O2, Valeric Acid, 102.13 g/mol
b. H3PO3, Phosphorous acid, 82 g/mol
7. For each compound, write the condensed formula, name the compound, and give its molar mass.
a. C2H5NH2, Ethylamine, 45.08 g/mol
b. HIO3, Iodic acid, 175.91 g/mol
8. Calculate the number of moles in 5.00 × 102 g of each substance. How many molecules or formula units are present in each sample?
a. 5.37 × 1024 mol
b. 3.01 × 1024 mol
c. 8.80 × 1023 mol
d. 4.04 × 1024 mol
e. 6.84 × 1024 mol
9. Calculate the mass in grams of each sample.
a. 47.85 grams
b. 384.52 grams
c. 536.57 grams
10. Give the number of molecules or formula units in each sample.
a. 7.83x1021 molecules
b. 6.20x1023 molecules
c. 1.60x1023 molecules
11. Give the number of moles in each sample.
a. 1590.8 moles
b. 6011.3 moles
c. 115244.1 moles
12. Solutions of iodine are used as antiseptics and disinfectants. How many iodine atoms correspond to 11.0 g of molecular iodine (I2)?
2.61 x1022 molecules
13. What is the total number of atoms in each sample?
a. 2.60x1023 atoms
b. 1.01x1025 atoms
c. 1.09x1023 atoms
d. 2.41x1024 atoms
14. What is the total number of atoms in each sample?
a. 5.9x1023 atoms
b. 2.8x1024 atoms
c. 3.31x1024 atoms
d. 8.15x1024 atoms
15. What is the total number of atoms in each sample?
a. 3.69x1022 atoms
b. 1.13x1023 atoms
c. 8.54x1022 atoms
d. 3.50x1023 atoms
16.
a. False, the number of molecules in 0.5 mol Cl2 are the same amount of molecules in H2
b. False, the number of molecules in H2 is 2 x (6.022 x10^23) H atoms
c. True, 2 H (1.01 amu) + 1 O (16.01) = 18.0 amu
d. True, C6H6 -> 12(6) + 1(6) = 78 amu
17. Complete the following table
a. 0.39
b. 2.36x10^23
c. 7.08x10^23
d. 482.8
e. 1.71x10^24
f. 8.55x10^24
g. 7932.7
h. 148.3
i. 5.36x10^26
j. 46938.5
k. 425.7
l. 1276.98
m. 126.5
n. 7.77x10^23
o. 5.44x10^24
p. 0.14
q. 8.27x10^22
r. 1.65x10^24
s. 34358
t. 406.8
u. 1.23x10^2
18. Give the formula mass or the molecular mass of each substance.
- 261.67 amu
- 301.04 amu
- 286.98 amu
- 551.73 amu
19. Give the formula mass or the molecular mass of each substance.
- 273.21 amu
- 69.62 amu
- 330.04 amu
- 426.99 amu
4.6-4.9: Percent Composition, Empirical Formula, and Molecular Formula
Conceptual Problems
- What is the relationship between an empirical formula and a molecular formula
- Construct a flowchart showing how you would determine the empirical formula of a compound from its percent composition.
Numerical Problems
1. What is the mass percentage of water in each hydrate?
a. H3AsO4·5H2O
b. NH4NiCl3·6H2O
c. Al(NO3)3·9H2O
2. What is the mass percentage of water in each hydrate?
a. CaSO4·2H2O
b. Fe(NO3)3·9H2O
c. (NH4)3ZrOH(CO3)3·2H2O
3. Which of the following has the greatest mass percentage of oxygen—KMnO4, K2Cr2O7, or Fe2O3?
4. Which of the following has the greatest mass percentage of oxygen—ThOCl2, MgCO3, or NO2Cl?
5. Calculate the percent composition of the element shown in bold in each compound.
a. SbBr3
b. As2I4
c. AlPO4
d. C6H10O
6. Calculate the percent composition of the element shown in bold in each compound.
a. HBrO3
b CsReO4
c. C3H8O
d. FeSO4
7. A sample of a chromium compound has a molar mass of 151.99 g/mol. Elemental analysis of the compound shows that it contains 68.43% chromium and 31.57% oxygen. What is the identity of the compound?
8. The percentages of iron and oxygen in the three most common binary compounds of iron and oxygen are given in the following table. Write the empirical formulas of these three compounds.
| Compound | % Iron | % Oxygen | Empirical Formula |
|---|---|---|---|
| 1 | 69.9 | 30.1 | |
| 2 | 77.7 | 22.3 | |
| 3 | 72.4 | 27.6 |
9. What is the mass percentage of water in each hydrate?
a. LiCl·H2O
b. MgSO4·7H2O
c. Sr(NO3)2·4H2O
10. What is the mass percentage of water in each hydrate?
a. CaHPO4·2H2O
b. FeCl2·4H2O
c. Mg(NO3)2·4H2O
11. Two hydrates were weighed, heated to drive off the waters of hydration, and then cooled. The residues were then reweighed. Based on the following results, what are the formulas of the hydrates?
| Compound | Initial Mass (g) | Mass after Cooling (g) |
|---|---|---|
| NiSO4·xH2O | 2.08 | 1.22 |
| CoCl2·xH2O | 1.62 | 0.88 |
12. Which contains the greatest mass percentage of sulfur—FeS2, Na2S2O4, or Na2S?
13. Given equal masses of each, which contains the greatest mass percentage of sulfur—NaHSO4 or K2SO4?
14. Calculate the mass percentage of oxygen in each polyatomic ion.
a. bicarbonate
b. chromate
c. acetate
d. sulfite
15. Calculate the mass percentage of oxygen in each polyatomic ion.
a. oxalate
b. nitrite
c. dihydrogen phosphate
d. thiocyanate
16. The empirical formula of garnet, a gemstone, is Fe3Al2Si3O12. An analysis of a sample of garnet gave a value of 13.8% for the mass percentage of silicon. Is this consistent with the empirical formula?
17. A compound has the empirical formula C2H4O, and its formula mass is 88 g. What is its molecular formula?
18. Mirex is an insecticide that contains 22.01% carbon and 77.99% chlorine. It has a molecular mass of 545.59 g. What is its empirical formula? What is its molecular formula?
27. Calculate the formula mass or the molecular mass of each compound.
a. MoCl5
b. B2O3
c. bromobenzene
d. cyclohexene
e. phosphoric acid
f. ethylamine
Conceptual Answers
1) What is the relationship between an empirical formula and a molecular formula
- An empirical formula refers to the simplest ratio of elements that is obtained from a chemical formula while a molecular formula is calculated to show the actual formula of a molecular compound.
2) Construct a flowchart showing how you would determine the empirical formula of a compound from its percent composition.
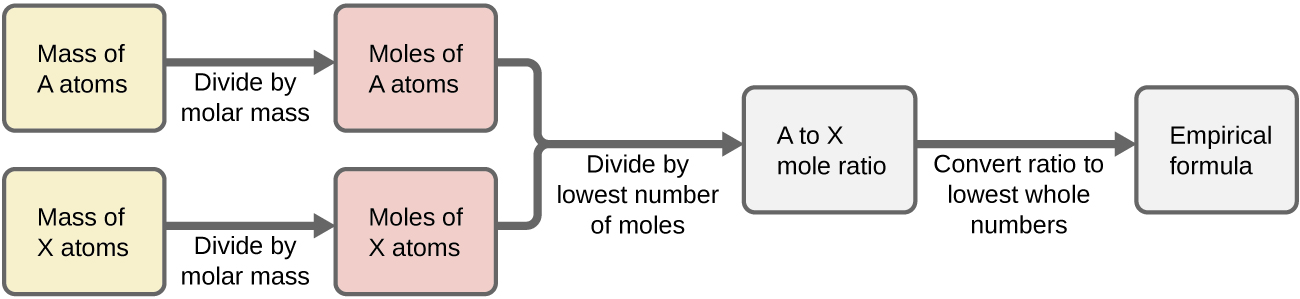
Numerical Answers
a. What is the formula mass of each species?
a. 53.49146 amu
b. 49.0072 amu
c. 58.3197 amu
d. 310.177 amu
e. 73.891 amu
f. 81.07 amu
b. What is the molecular or formula mass of each compound?
a.158.034 amu
b. 142.04 amu
c. 27.0253 amu
d. 97.181 amu
e. 124.1 amu
f. 65.99 amu
1. To two decimal places, the percentages are:
a. 5.97%
b. 37.12%
c. 43.22%
2. Percentage of Oxygen in each hydrates are:
a. 20.93%
b. 40.13%
c. 9.52%
3. % oxygen: KMnO4, 40.50%; K2Cr2O7, 38.07%; Fe2O3, 30.06%
4. % oxygen: ThOCl2, 5.02%; MgCO3, 56.93%; NO2Cl, 39.28%
5. To two decimal places, the percentages are:
a. 66.32% Br
b. 22.79% As
c. 25.40% P
d. 73.43% C
6.
a. 61.98% Br
b. 34.69% Cs
c. 59.96% C
d. 21.11% S
7. Cr2O3.
8. Empirical Formulas
- Fe2O3
- Fe4O4
- Fe6O8
9. To two decimal places, the percentages are:
a. 29.82%
b. 51.16%
c. 25.40%
10. What is the mass percentage of water in each hydrate?
a. 20.94%
b. 36.25%
c. 32.70%
11. NiSO4 · 6H2O and CoCl2 · 6H2O
12. FeS2
13. NaHSO4
14. Calculate the mass percentage of oxygen in each polyatomic ion.
a. 78.66%
b. 55.17%
c. 54.19%
d. 59.95%
15.
a. 72.71%
b. 69.55%
c. 65.99%
d. 0%
16. The empirical formula of garnet, a gemstone, is Fe3Al2Si3O12. An analysis of a sample of garnet gave a value of 13.8% for the mass percentage of silicon. Is this consistent with the empirical formula?
No, the calculated mass percentage of silicon in garnet is 16.93%
17. C4H8O2
18.
Empirical Formula: C10Cl12
Molecular Formula: C10Cl12
19. How many moles of CO2 and H2O will be produced by combustion analysis of 0.010 mol of styrene?
Moles of CO2: 0.08 mol CO2
Moles of H2O: 0.04 mol H2O
20. How many moles of CO2, H2O, and N2 will be produced by combustion analysis of 0.0080 mol of aniline?
Mole of CO2: 0.048 mol CO2
Mole of H2O: 0.028 mol H2O
Mole of N2: 0.004 mol N2
21. How many moles of CO2, H2O, and N2 will be produced by combustion analysis of 0.0074 mol of aspartame?
Mole of CO2: 0.104 mol CO2
Mole of H2O: 0.666 mol H2O
Mole of N2: 0.0074 mol N2
22. How many moles of CO2, H2O, N2, and SO2 will be produced by combustion analysis of 0.0060 mol of penicillin G?
Mole of CO2: 0.096 mol CO2
Mole of H2O: 0.054 mol H2O
Mole of N2: 0.060 mol N2
Mole of SO2: 0.060 mol SO2
23.
a. 27.6 mg C and 1.98 mg H
b. 5.2 mg O
c. 15%
d. C7H6O
e. C7H6O
24. Salicylic acid is used to make aspirin. It contains only carbon, oxygen, and hydrogen. Combustion of a 43.5 mg sample of this compound produced 97.1 mg of CO2 and 17.0 mg of H2O.
a. What is the mass of oxygen in the sample?
70.4mg
b. What is the mass percentage of oxygen in the sample?
61.70%
c. What is the empirical formula of salicylic acid?
C7H6O3
d. The molar mass of salicylic acid is 138.12 g/mol. What is its molecular formula?
C7H6O3
25. hydrocyanic acid, HCN
26. Calculate the formula mass or the molecular mass of each compound.
a. 130.1849 amu
b. 60.1 amu
c. 158.034 amu
d. 323.4 amu
e. 82.07 amu
f. 106.17 amu
27. To two decimal places, the values are:
a. 273.23 amu
b. 69.62 amu
c. 157.01 amu
d. 82.14 amu
e. 98.00 amu
f. 45.08 amu
28. Cyclobutene
29. Urea