13.10: Polymerization Reactions
- Page ID
- 476657
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain the difference between an addition polymer and a condensation polymer.
We reap the benefits of using Styrofoam containers, but do not often consider where they end up. Styrofoam materials do not break down quickly under exposure to the elements. When buried in a landfill, styrofoam will remain intact for a long time. The good news is that there is not a lot of this pollutant found in landfills (maybe about \(0.5\%\) by weight of the total mass of garbage). There is no good way to recycle Styrofoam at present, but in the future, a creative scientist may change that. Styrofoam is an example of a polymer.
Polymers are very different from the other kinds of organic molecules that you have seen so far. Whereas other compounds are of relatively low molar mass, polymers are giant molecules of very high molar mass. Polymers are the primary components of all sorts of plastics and related compounds. A polymer is a large molecule formed of many smaller molecules covalently bonded in a repeating pattern. The small molecules which make up the polymer are called monomers. Polymers generally form either from an addition reaction or a condensation reaction. The "small molecules" that polymers are comprised of are often much more complicated than the compounds we have been exploring earlier in this chapter. We will not get into the details of these more advanced structures in this text, but will provide a few examples of types of polymer reactions.
Addition Polymers
An addition polymer is a polymer formed by chain addition reactions between monomers that contain a double bond. Molecules of ethene can polymerize with each other under the right conditions to form the polymer called polyethylene.
\[n \ce{CH_2=CH_2} \rightarrow \ce{-(CH_2CH_2)}_n-\nonumber \]
The letter \(n\) stands for the number of monomers that are joined in repeated fashion to make the polymer, and can have a value in the hundreds or even thousands.
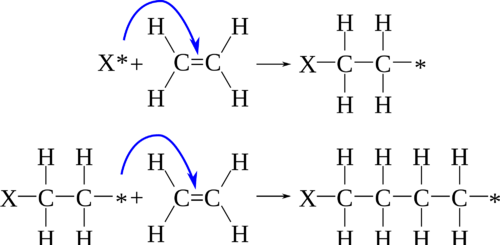
Polyethylene can have different properties depending on the length of the polymer chains, and on how efficiently they pack together. Some common products made from different forms of polyethylene include plastic bottles, plastic bags, and harder plastic objects such as milk crates.
Several other kinds of unsaturated monomers can be polymerized, and are components in common household products. Polypropylene is stiffer than polyethylene, and is in plastic utensils and some other types of containers.
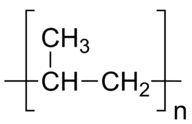
Polystyrene is used in insulation and in molded items such as coffee cups.

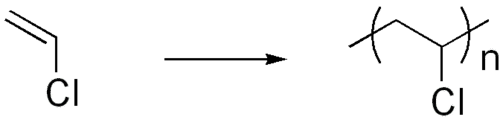
Polyvinyl chloride (PVC) is extensively used for plumbing pipes.
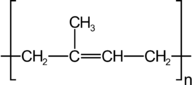
Polyisoprene is a polymer of isoprene and is better known as rubber. It is produced naturally by rubber trees, but several variants have been developed which demonstrate improvements on the properties of natural rubber.
Condensation Polymers
A condensation polymer is a polymer formed by condensation reactions. Monomers of condensation polymers must contain two functional groups so that each monomer can link up with two other monomers. One type of condensation polymer is called a polyamide. An amide is characterized by the functional group shown below wherein the carbon of a carbonyl group is bonded to the nitrogen of an amine.
One pair of monomers that can form a polyamide is that of adipic acid and hexanediamine. Adipic acid is a carboxylic acid with two carboxyl groups on either end of the molecule. Hexanediamine has amino groups on either end of a six-carbon chain. When these molecules react with each other, a molecule of water is eliminated, classifying it as a condensation reaction (see figure below).
The polymer that results from the repetition of the condensation reaction is a polyamide called nylon-66. Nylon-66 was first invented in 1935 and has been used in all sorts of products. Polyamides, including Nylon-66, are commonly found in fibers and clothing, cooking utensils, fishing line, and carpeting—among many other applications.
Polyester is another common type of condensation polymer. Recall that esters are formed from the reaction of an alcohol with a carboxylic acid. When both the acid and alcohol have two functional groups, the ester is capable of being polymerized. One such polyester is called polyethylene terephthalate (PET) and is formed from the reaction of ethylene glycol with terephthalic acid. The structure of PET is shown below.
PET is used in tires, photographic film, food packaging, and clothing. Polyester fabric is used in permanent-press clothing. Its resistance to wrinkling comes from the cross-linking of the polymer strands.
Section Summary
- A polymer is a large molecule formed of many smaller molecules covalently bonded in a repeating pattern; they are the primary components of all sorts of plastics and related compounds.
- The small molecules which make up polymers are called monomers.
- Polymers generally form either from an addition reaction or a condensation reaction.
- An addition polymer is a polymer formed by chain addition reactions between monomers that contain a double bond.
- A condensation polymer is a polymer formed by condensation reactions which release a small molecule such as water.
Glossary
- polymer
- A large molecule formed of many smaller molecules covalently bonded in a repeating pattern.
- monomers
- The small molecules which make up a polymer.
- addition polymer
- A polymer formed by chain addition reactions between monomers that contain a double bond.
- condensation polymer
- a polymer formed by condensation reactions, such as a reaction that forms water molecules as a side product.


