13.5: Energy and Chemical Reactions
- Page ID
- 476637
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
- Describe how energy might interact with a chemical reaction, both graphically and in words.
Gunpowder was originally developed by the Chinese in the ninth century CE, primarily for rockets. This material is composed of charcoal, sulfur, and saltpeter (potassium nitrate). The explosive reaction that occurs involves the conversion of the charcoal to carbon dioxide, with the potassium nitrate providing the extra oxygen needed for a rapid reaction. Although sulfur was included to stabilize the product, gunpowder is still highly explosive.
Chemical Potential Energy
As was discussed earlier in this text, energy is the capacity for doing work or supplying heat. When you fill your car with gasoline, you are providing it with potential energy. Chemical potential energy is the energy stored in the chemical bonds of a substance. The various chemicals that make up gasoline contain a large amount of chemical potential energy that is released when the gasoline is burned in a controlled way in the engine of the car. The release of that energy does two things: some of the potential energy is transformed into work, which is used to move the car; at the same time, some of the potential energy is converted to heat and makes the car's engine very hot. The energy changes of a system occur as either heat or work, or some combination of both.

Dynamite is another example of chemical potential energy. The major component of dynamite is nitroglycerin, a very unstable material. By mixing it with diatomaceous earth, the stability is increased and it is less likely to explode if it receives a physical shock. When ignited, the nitroglycerin explodes rapidly, releasing large amounts of nitrogen and other gases along with a massive amount of heat.

Exothermic and Endothermic Processes
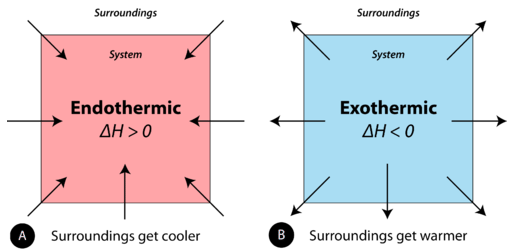
When physical or chemical changes occur, they are generally accompanied by a transfer of energy. The law of conservation of energy states that in any physical or chemical process, energy is neither created nor destroyed. In other words, the entire energy in the universe is conserved. In order to better understand the energy changes taking place during a reaction, we need to define two parts of the universe: the system and the surroundings. The system is the specific portion of matter in a given space that is being studied during an experiment or an observation. The surroundings is everything in the universe that is not part of the system. In practical terms for a laboratory chemist, the system is the particular chemicals being reacted, while the surroundings is the immediate vicinity within the room. During most processes, energy is exchanged between the system and the surroundings. If the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings.
A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an endothermic process, the system gains heat from the surroundings, and so the temperature of the surroundings decreases. The quantity of heat for a process is represented by the letter \(q\). The sign of \(q\) for an endothermic process is positive because the system is gaining heat. A chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Because the surroundings are gaining heat from the system, the temperature of the surroundings increases. The sign of \(q\) for an exothermic process is negative because the system is losing heat.
Scientists have define a quantity called enthalpy to make it more useful to keep track of the flow of heat in a reaction from a thermodynamics perspective. The definition of enthalpy is beyond the scope of this text, but it is defined in such a way that the heat flow at constant pressure is equal to the change in enthalpy (represented as \(\left( \Delta H \right)\). Most reactions of interest to chemists occur at constant pressure, so this creates a convenient way of taking a non-conservative property such a heat flow and equating it with a state function such as enthalpy, which is more convenient mathematically. Throughout the rest of this text we will refer to change in enthalpy, \(\left( \Delta H \right)\), and heat flow somewhat interchangeably.
Activation Energy

Why do some chemical reactions occur readily while others require input of heat in order to take place? If we mix metallic sodium with water, a reaction occurs immediately, releasing a great deal of heat (enough to ignite the hydrogen gas that is formed). Group II metals, such as calcium, react at a much slower rate. Unlike the extremely vigorous reaction with sodium, the reaction with calcium is slow enough that we can trap the hydrogen gas released.
Supplying reactant particles with energy causes the bonds between the atoms to vibrate with a greater frequency. This increase in vibrational energy makes a chemical bond more likely to break, and a chemical reaction more likely to occur, when those particles collide with other particles. The activation energy for a reaction is the minimum energy that colliding particles must have in order to undergo a reaction. Some reactions occur readily at room temperature because the reacting particles already have the requisite activation energy at that temperature. Other reactions only occur when heated because the particles do not have enough energy unless an external source of heat provides the particles with more kinetic energy.
Potential Energy Diagrams
The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams. The total potential energy of the system increases for the endothermic reaction as the system absorbs energy from the surroundings. The total potential energy of the system decreases for the exothermic reaction as the system releases energy to the surroundings.

The activation energy for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products. For this reason, the activation energy of a reaction is sometimes referred to as the activation energy barrier. Reacting particles must have enough energy so that when they collide, they can overcome that barrier (see figure below).

Section Summary
- Chemical potential energy is energy available in the chemical bonds of a compound.
- A potential energy diagram shows the change in potential energy of a system as reactants are converted into products.
- Potential energy diagrams for endothermic and exothermic reactions are described.
- Diagrams of activation energy and reaction progress are given.
Glossary
- chemical potential energy
- The energy stored in the chemical bonds of a substance.
- system
- The specific portion of matter in a given space that is being studied during an experiment or an observation.
- surroundings
- Everything in the universe that is not part of the system.
- endothermic
- A chemical or physical change in which heat is absorbed by the system from the surroundings.
- exothermic
- A chemical or physical change in which heat is released by the system into the surroundings.
- activation energy
- The minimum energy that colliding particles must have in order to undergo a reaction.


