11.9: Intermolecular Forces
- Page ID
- 476594
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain the difference between dipole-dipole forces, dispersion forces, and hydrogen bonds
Molecules are attracted to each other, but not as strongly as a chemical bond such as an ionic bond. However these attractions are important as we will see. In this section we will discuss some different forms of these attractions, which are called intermolecular forces.
Dipole-Dipole Forces
Dipole-dipole forces are the attractive forces that occur between polar molecules. A molecule of hydrogen chloride has a partially positive hydrogen atom and a partially negative chlorine atom. In a collection of many hydrogen chloride molecules, the molecules will align themselves so that the oppositely charged regions of neighboring molecules are near each other.
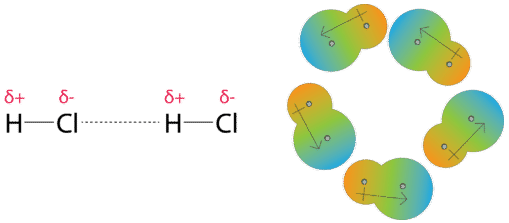
Dipole-dipole forces are similar in nature to ionic bonds, but much weaker.
London Dispersion Forces
Dispersion forces are also considered a type of van der Waals force and are the weakest of all intermolecular forces. They are often called London dispersion forces after Fritz London (1900-1954), who first proposed their existence in 1930. London dispersion forces are the intermolecular forces that occur between atoms, and between nonpolar molecules as a result of the motion of electrons.
The electron cloud of a helium atom contains two electrons, which can normally be expected to be equally distributed spatially around the nucleus. However, at any given moment the electron distribution may be uneven, resulting in an instantaneous dipole. This weak and temporary dipole subsequently influences neighboring helium atoms through electrostatic attraction and repulsion. It induces a dipole on nearby helium atoms (see figure below).
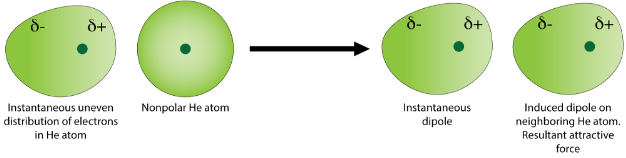
The instantaneous and induced dipoles are weakly attracted to one another. The strength of dispersion forces increases as the number of electrons in the atoms or nonpolar molecules increases.
The halogen group consists of four elements that all take the form of nonpolar diatomic molecules. The table below shows a comparison of the melting and boiling points for each.
| Table \(\PageIndex{1}\): Melting and Boiling Points of Halogens | ||||
|---|---|---|---|---|
| Molecule | Total Number of Electrons | Melting Point \(\left( ^\text{o} \text{C} \right)\) | Boiling Point \(\left( ^\text{o} \text{C} \right)\) | Physical State at Room Temperature |
| \(\ce{F_2}\) | 18 | -220 | -188 | gas |
| \(\ce{Cl_2}\) | 34 | -102 | -34 | gas |
| \(\ce{Br_2}\) | 70 | -7 | 59 | liquid |
| \(\ce{I_2}\) | 106 | 114 | 184 | solid |
The dispersion forces are strongest for iodine molecules because they have the greatest number of electrons. The relatively stronger forces result in melting and boiling points that are the highest of the halogen group. These forces are strong enough to hold iodine molecules close together in the solid state at room temperature. The dispersion forces are progressively weaker for bromine, chloride, and fluorine; this is illustrated in their steadily lower melting and boiling points. Bromine is a liquid at room temperature, while chlorine and fluorine are gases whose molecules are much further apart from one another. Intermolecular forces are nearly nonexistent in the gas state, and so the dispersion forces in chlorine and fluorine only become measurable as the temperature decreases and they condense into the liquid state.
Hydrogen Bonding
The attractive force between water molecules is a dipole interaction. The hydrogen atoms are bound to the highly electronegative oxygen atom (which also possesses two lone pair sets of electrons, making for a very polar bond). The partially positive hydrogen atom of one molecule is then attracted to the oxygen atom of a nearby water molecule (see figure below).
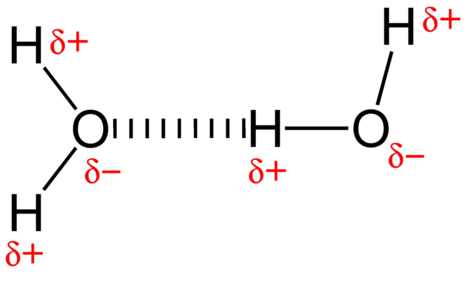
A hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small, highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring molecule. Hydrogen bonds are very strong compared to other dipole interactions. The strength of a typical hydrogen bond is about \(5\%\) of that of a covalent bond.
Hydrogen bonding occurs only in molecules where hydrogen is covalently bonded to one of three elements: fluorine, oxygen, or nitrogen. These three elements are so electronegative that they withdraw the majority of the electron density in the covalent bond with hydrogen, leaving the \(\ce{H}\) atom very electron-deficient. The \(\ce{H}\) atom nearly acts as a bare proton, leaving it very attracted to lone pair electrons on a nearby atom.
The hydrogen bonding that occurs in water leads to some unusual, but very important, properties. Most molecular compounds that have a mass similar to water are gases at room temperature. Because of the strong hydrogen bonds, water molecules are able to stay condensed in the liquid state. The figure below shows how the bent shape, and two hydrogen atoms per molecule, allows each water molecule to be able to hydrogen bond to two other molecules.
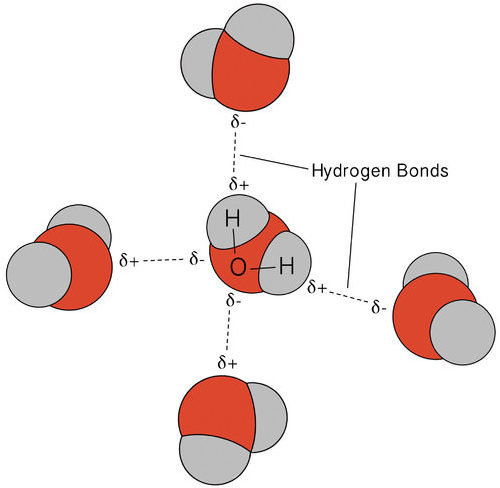
In the liquid state, the hydrogen bonds of water can break and reform as the molecules flow from one place to another. When water is cooled, the molecules begin to slow down. Eventually, when water is frozen to ice, the hydrogen bonds become permanent and form a very specific network (see figure below).

The bent shape of the molecules leads to gaps in the hydrogen bonding network of ice. Ice has a very unusual property—its solid state is less dense than its liquid state. Ice floats in water. Virtually all other substances are denser in the solid state than in the liquid state. Hydrogen bonds play a very important biological role in the physical structures of proteins and nucleic acids.
Section Summary
- Van der Waals forces are weak interactions between molecules that involve dipoles.
- Polar molecules have permanent dipole-dipole interactions.
- Nonpolar molecules can interact by way of London dispersion forces.
- Hydrogen bonds form when a \(\ce{H}\) attached to a \(\ce{N}\), \(\ce{O}\), or \(\ce{F}\) atom interacts with another \(\ce{N}\), \(\ce{O}\), or \(\ce{F}\) atom.
Glossary
- dipole-dipole forces
- The attractive forces that occur between polar molecules.
- dispersion forces
- The intermolecular forces that occur between atoms, and between nonpolar molecules as a result of the motion of electrons.
- hydrogen bond
- an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small, highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring molecule.
- intermolecular forces
- Attractions between molecules which are not as strong as a chemical bond.


