10.9: Electron Shells
- Page ID
- 477216
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain the trend of filling energy levels with electrons.
- Explain the connection between valence electrons and the periodic law.
From both the Bohr model and quantum mechanics we now know that electrons are arranged in an orderly manner of energy levels. In this final section, we will see how this particular arrangement of electrons relates back to the periodic law.
Electron Shells
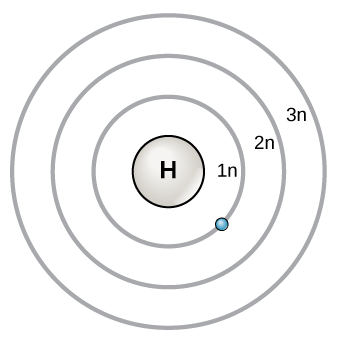
The energy levels for the electrons in an atom are often referred to as electron shells. (Note: as we discussed previously, this does not refer to movement according to classical mechanics, but an indication of increasing potential energy as the shell number increases.) These shells are given notations 1n, 2n, etc. to indicate the increasing potential energy of each shell, as indicated in Figure \(\PageIndex{1}\) Each of these energy levels contains one or more orbitals that have a limited number of electrons that they may hold. The electrons are generally added to the lowest energy orbital available. When the available orbitals within one energy level have been filled, any additional electrons must be added to the next energy level. In addition to being of a higher potential energy, each successive energy level has a greater number of orbitals it contains, and therefore a greater capacity to contain electrons. As the number of orbitals within an energy level increases, there is a bit more complexity to the order in which the electrons are added. The details of this complexity are beyond the scope of this course, but we will mention some of their implications as it relates to the effects this has on periodic trends.
When we begin to add these electrons to an energy level, we start to see a trend emerge that mirrors the trend first observed by Mendeleev. At some point an electron shell will be filled with electrons. Regardless of which electron shell we are talking about, the significance of a filled electron shell has vast implications for the chemical properties of that atom. If we imagine an atom with one additional electron, we end up with an atom that has a single electron in its outer electron shell. Likewise, the significance of an electron shell with a single electron has vast implications on the chemical properties of that atom. Figure \(\PageIndex{2}\) shows models of these electron shells for a portion of the periodic table. Note that each element in group 1 has a single electron in its outer energy shell, and that each element in group 18 has that energy shell filled (though the number of electrons necessary to fill it changes from 2 to 8 after the first shell). Also note that groups 14 and 17 have identical numbers of electrons in their outer energy level. This trend is so significant that we have a special name for the electrons in the outermost electron shell: valence electrons. It turns out that most of the chemical properties of greatest importance to the function of life occur because of the behavior of these electrons. We shall explore the behavior of these electrons in more detail later in this text.

Valence Electrons and the Periodic Law
It turns out that the reason why the periodic law works as a predictor of chemical properties is because of the behavior of the valence electrons. If we look at a periodic table, such as the one shown in Figure \(\PageIndex{3}\), we can see how some of these trends emerge. The groups which have the greatest similarities in chemical properties were the ones to which we ascribed special names: alkali metals, alkaline earth metals, halogens, and noble gases. Note that these are the positions on the periodic table where the valence electron shell is full, nearly full, or nearly empty. The tendency towards a full valence electron shell is so important that the elements within each of these groups become almost identical in their chemical properties. Elsewhere on the periodic table these trends exist, but are not as pronounced. In fact the areas of the period table we had designated as blocks even though they encompassed several groups (transition metals, lanthanides, actinides) have more complex chemistry because it sometimes involves electrons that are not in the valence shell.

Ironically, Mendeleev never actually believed in electrons, even though they were the factor that could explain the natural law he had discovered! This is often an unfortunate trend among established scientists who are not able to recognize the contributions of those who come along after them. Nevertheless the progress of science has continued to move along. However, it is an important life lesson to realize that even as teachers there are things we might learn from our students.
Section Summary
- Energy levels are predicted by both the Bohr model and quantum mechanics.
- Electrons typically fill orbitals of the highest vacant energy level.
- Electrons in the outermost energy level are called valence electrons.
- Valence electrons predict chemical properties, and thereby the periodic law.
Glossary
- electron shells
- the energy levels of the electrons within an atom.
- orbitals
- most precise information typically available about the energy and location of an electron. Capable of containing up to two electrons.
- valence electrons
- electrons found in the outermost electron shell.
Contributors and Attributions
- Jamie MacArthur
- Biology. Provided by: OpenStax CNX. License: CC BY: Attribution(opens in new window). License Terms: Download for free


