9.7: Our Extraordinary World of Colors
- Page ID
- 477134
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Know how the following factors contribute to our sense of color: absorption, transmission, emission, additive mixing, and subtractive mixing.
- Know how the structure of our eyes affects our interpretations of color.
- Know how these factors affect our perceptions of natural phenomena such as the color of the ocean, the sky and sunsets.
We have said a lot about how light can and does interact with matter, but how does that result in the colorful world that we live in? Why is the sky blue? Why are sunsets red? We now have the background knowledge to answer these kinds of questions that could be very important to people we meet in our lives.
The visible light portion of the electromagnetic spectrum has wavelengths in approximately (because everyone's vision is a little bit different) the 400-700 nanometer range (a nanometer, “nm,” is 10-9 meters). Each specific wavelength corresponds to a different color (Figure \(\PageIndex{1}\)), and when all these wavelengths are present, it appears to us as white light. It turns out that for the light from the Sun and the light from the lamps in your house, the number of wavelengths emitted is essentially infinite. This is why it is called a spectrum. Where does red end and orange begin? From a scientific perspective, we can measure a specific wavelength and agree on a convention for the naming of that color. As we shall see later in this text, sometimes knowing the specifics of a wavelength of light can be very important. But most of our interactions with light are not scientific, they are aesthetic. When is something green or blue or cyan or teal? It is a very subjective decision, and there have probably been many arguments about it. Different cultures throughout time have defined color differently based on what was important to them. At the time that much of the work on optics was being done the number seven and the color indigo were very important. Hence we have the conventional colors of red orange yellow green blue indigo violet. And depending on the source, sometimes either the indigo or violet are left out.

Absorption and Transmission and Color
Color is perceived in two ways, through additive mixing, where different colors are made by combining different colors of light, and through subtractive mixing, where different wavelengths of light are taken out so that the light is no longer pure white. As shown in Figure \(\PageIndex{2}\), the idea behind subtractive mixing is that white light (which is made from all the colors mixed together) interacts with an object. The object absorbs some of the light, and then reflects or transmits (or both, depending on the object) the rest of the light, which contacts the eye. The object is perceived as whichever color is not absorbed. In Figure \(\PageIndex{2}\), white light (simplified as green, red, and blue bands) is shone through a solution. The solution absorbs the red and green wavelengths; however, the blue light is reflected and passes through, so the solution appears blue. (This is why large bodies of water, such as the ocean, appear blue. The bluish color of water is too faint to observe in the smaller quantities of water we see in our drinking glasses, for example.) This procedure takes place whenever an object displays visible color. If none of the light is absorbed, and all is reflected back off, the object appears white; if all of the light is absorbed, and there is none left to reflect or transmit through, the object appears black. This is why white objects do not get as hot when exposed to sunlight as black objects.
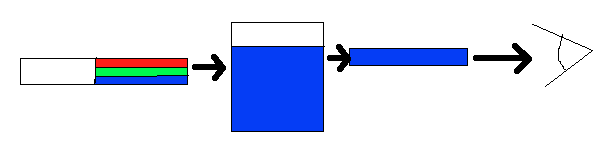
Complimentary Colors
Additive mixing is when colors are mixed together to produce a new color. If we combine red, green, and blue, as in our last example, we get white light. There are other combinations that will produce other colors, as you may have learned in art class. It turns out that for the eyes of humans we have receptors which are specifically geared towards red, green, and blue colors. We interpret other colors as mixtures of these three based on how our eyes work. Those other colors do still exist in the world: we know this from the wavelengths of the spectrum.
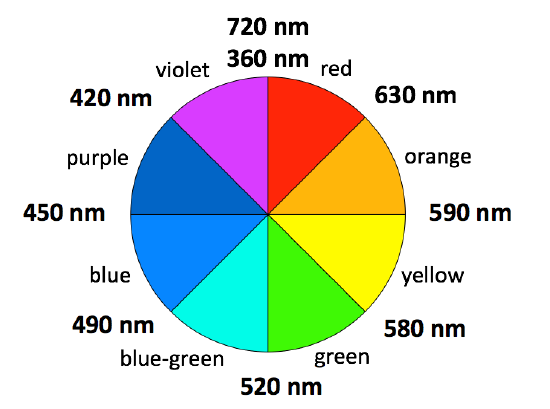
A color wheel shows the complimentary color for any other color. The complimentary color is the color that will make white light if mixed together. A color wheel is shown in figure \(\PageIndex{3}\). The color directly across from a given color is its complimentary color. Another way of understanding the color we see for a substance related to absorption of light is that we will see it as the complimentary color to the color of light that is most strongly absorbed. Most objects will absorb light of a variety of different wavelengths, giving rise to a variety of different colors.
Factors Affecting What We See in the World
In addition to the properties of light, the properties of our eyes will determine what we are able to see. We already mentioned that our eyes have receptors to red, green, and blue and interpret other colors as combinations of how these receptors interact with the light we see. It also turns out that our eyes are most receptive to yellow light, which happens to be the highest intensity wavelength of light coming from the Sun. As we move away from yellow towards the other colors in the visible range, our ability to see those colors decreases.
Sometimes matter will absorb some forms of radiation but be transparent to others. When there are some wavelengths of visible light that are absorbed and others that are not, this affects the color that we perceive when we look at the object. But if no light is there to impinge on the object, then it is invisible to us. If an object is emitting light, such as the Sun or a lamp, the color of the wavelengths emitted by that object is the color that we see. If it is not emitting that light, then we see the color that it transmits. And there are even more complicated interactions, such as a light that emits only certain wavelengths impinging on an object.
Another factor that we need to consider related to the color of some objects is the scattering of light. As we discussed previously, different wavelengths of light are bent to different extents when they interact with various forms of matter. This gives rise to the rainbows we see when light from the sun interacts with a drop of water, as we had previously discussed. But what happens when there are no raindrops to interact with? In this case, the light from the Sun interacts with what will always be present in our atmosphere: the atoms of the air. These atoms absorb the light from the Sun and re-emit it. Just as when light travels through any substance, the amount each of the wavelengths are bent will vary. It turns out that violet light is bent the most and red light is bent the least. This means that the violet light is scattered the most. So why does the sky not appear violet? This is mostly because our eyes do not see violet as well. Blue is the color where the degree of scattering and the degree to which our eyes can properly interpret the light match up. The shading of the blue is also affected by how much scattering is occurring in the air. If there is a lot of moisture or particles in the air, it will be a lighter blue, as other interactions mean there are more colors mixed in with it. If the air is very dry, it will be a darker blue, perhaps some people can even see some of it as violet.
Then why are sunsets red? Red is the color that is the least scattered by the atoms of the atmosphere. The more atmosphere that the sunlight has to travel through, the more each of the other colors will be scattered. Sunlight has to travel through the greatest amount of atmosphere at sunrise and sunset. In the middle of the day, the sun is directly overhead and it has the fewest number of atoms for the sunlight to interact with, but at the beginning and end of the day there are more atoms, hence the reddish color of the sky around the sun at both sunrise and sunset.
Section Summary
- The visible portion of the spectrum occurs for electromagnetic radiation with a wavelength between 400 and 700 nm, with colors closer to 400 appearing more violet and transitioning through all of the other visible colors to red before reaching 700.
- When a substance absorbs some wavelengths of light but not others, we see that substance as the colors which were not absorbed, but transmitted. This is called subtractive mixing.
- Additive mixing is when different wavelengths of light are mixed and appear as a different color. Complimentary colors are the ones which create white when they are mixed additively.
- Our eyesight is most receptive to yellow light and is made of receptors that respond to blue, green, and red light. We have difficulty perceiving violet.
- The blue color of the sky and the red color of sunrises and sunsets are observed due to the scattering of light by the atoms in the atmosphere.
Contributors and Attributions
- Jamie MacArthur
- Deyu Wang (UCD)


