1.5.2: Fields of Science
- Page ID
- 477444
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Know the subject matter field for physics, chemistry, earth science, and astronomy.
Science has become so vast that it now includes many separate fields of study, each of which contain their own sub-disciplines. This course is focused on physical sciences, which are natural sciences focused on non-living things. We will provide a brief summary of some of these disciplines. This text will primarily focus on the first two of these: physics and chemistry. The other two, earth science and astronomy, depend on an understanding of chemistry and physics.
Physics
Science consists of the theories and laws that are the general truths of nature as well as the body of knowledge they encompass. Scientists are continually trying to expand this body of knowledge and to perfect the expression of the laws that describe it. Physics is concerned with describing the interactions of energy, matter, space, and time, and it is especially interested in what fundamental mechanisms underlie every phenomenon. The concern for describing the basic phenomena in nature essentially defines the realm of physics.
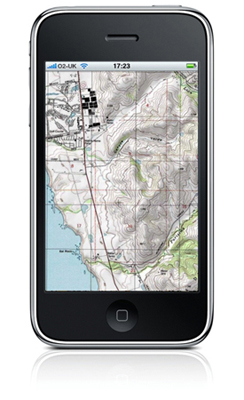
Physics aims to describe the function of everything around us, from the movement of tiny charged particles to the motion of people, cars, and spaceships. In fact, almost everything around you can be described quite accurately by the laws of physics. Consider a smart phone as in Figure \(\PageIndex{1}\): Physics describes how electricity interacts with the various circuits inside the device. This knowledge helps engineers select the appropriate materials and circuit layout when building the smart phone. Next, consider a Global Positioning System (GPS). Physics describes the relationship between the speed of an object, the distance over which it travels, and the time it takes to travel that distance. When you use a GPS device in a vehicle, it utilizes these physics equations to determine the travel time from one location to another.
Chemistry
Chemistry is the study of the composition of matter and the changes that matter undergoes. Matter is anything that has mass and takes up space. Virtually everything around us is matter, including both living and nonliving things. Chemistry affects nearly everything we see and every action we take. Chemistry explains why milk that is left in the refrigerator for too long turns sour. Chemistry explains why certain pollutants called chlorofluorocarbons have done lasting damage to the ozone layer of our planet. Chemistry explains why the leaves of deciduous trees turn from green in the summer to various shades of red and yellow in the autumn Figure \(\PageIndex{2}\).
Chemistry touches every area of our lives. The medicines we take, the food we eat, the clothes we wear—all of these materials and more are, in some way or another, products of chemistry.
Chemists look at the world in two ways, often simultaneously. The two worlds of the chemist are the macroscopic world and the microscopic world. Macroscopic refers to substances and objects that can be seen, touched, and measured directly. Microscopic refers to the small particles that make up all matter. Chemists must observe matter and do experiments macroscopically; then make generalizations and propose explanations that are microscopic in nature. For example, anyone can observe the physical change in appearance that occurs as an iron object, such as a tractor, is left out in the elements and gradually turns to rust. However, a chemist looking at the rusting tractor considers the individual atoms that make up the iron, and how they are changing as a result of exposure to oxygen in the air, and water from rain. Throughout the study of chemistry, there is often a switch back and forth between the macroscopic and microscopic worlds.
Earth Science
Earth is the mighty planet upon which we all live. Only recently have humans begun to understand the complexity of this planet. In fact, it was only a few hundred years ago that we discovered that Earth was just a tiny part of an enormous galaxy, which in turn is a small part of an even greater universe. Earth Science deals with any and all aspects of the Earth. Our Earth has molten lava, icy mountain peaks, steep canyons and towering waterfalls. Earth scientists study the atmosphere high above us as well as the planet’s core far beneath us. Earth scientists study parts of the Earth as big as continents and as small as the tiniest atom. In all its wonder, Earth scientists seek to understand the beautiful sphere on which we thrive (Figure \(\PageIndex{3}\)).
Because the Earth is so large and science is so complex, Earth scientists specialize in studying just a small aspect of our Earth. Since all of the branches are connected together, specialists work together to answer complicated questions. Important branches of Earth Science include geology, oceanography, climatology, and meteorology.
Astronomy
Astronomers have proven that our Earth and solar system are not the only set of planets in the universe. As of June 2015, over a thousand planets outside our solar system had been discovered. Although no one can be sure how many there are, astronomers estimate that there are billions of other planets. In addition, the universe contains black holes, other galaxies, asteroids, comets, and nebula. As big as Earth seems to us, the entire universe is vastly greater. Our Earth is an infinitesimally small part of our universe.
Astronomers use resources on the Earth to study physical things beyond the Earth. They use a variety of instruments like optical telescopes and radio telescopes to see things far beyond what the human eye can see. Spacecraft travel great distances in space to send us information on faraway places, while telescopes in orbit observe astronomical bodies from the darkness of space (Figure \(\PageIndex{4}\)).
Astronomers ask a wide variety of questions. Astronomers could study how an object or energy outside of Earth could affect us. An impact from an asteroid could have terrible effects for life on Earth. Strong bursts of energy from the sun, called solar flares, can knock out a power grid or disturb radio, television or cell phone communications. But astronomers ask bigger questions too. How was the universe created? Are there other planets on which we might live? Are there resources that we could use? Is there other life out there? Astronomy also relies on Earth Science, when scientists compare what we know about life on Earth to the chances of finding life beyond this planet.
Section Summary
- Physics is concerned with describing the interactions of energy, matter, space, and time, and it is especially interested in what fundamental mechanisms underlie every phenomenon.
- Chemistry is the study of the composition of matter and the changes that matter undergoes.
- Earth Science deals with any and all aspects of the Earth.
- Astronomy is the study of the universe beyond the Earth.
Contributions and Attributions
This page was curated by Jamie MacArthur (Madera Community College) using resources from the following locations.


