1.3.1: Laws and Theories in Science
- Page ID
- 477913
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify examples of laws and theories in science.
We have already discussed some of the methods of science, but what is the end result of science? For a single scientist, the end result would be a collection of all of the work they have done over their career, which may have variable results. For the scientific community as a whole, the end result is often going to be laws, theories, and principles. Although there are many scientific laws which bear the name of a single researcher, it is never really a single person who discovers anything, but the scientific community as a whole. (In some fields this is recognized by including multiple contributors in the naming of a phenomena. For example: the Soave-Redlich-Kwong equation of state is used in thermodynamics.) This can be no better epitomized than by considering the words of Isaac Newton, for whom three of the most important laws of physics have been named: "if I have seen further....it is by standing on the shoulders of giants."
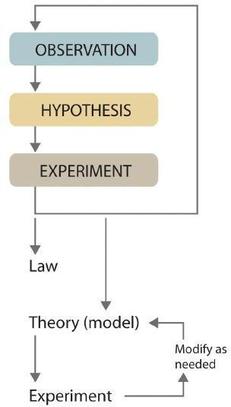
Scientists search for answers to questions and solutions to problems by using a procedure called the scientific method, as discussed previously. This procedure consists of making observations, formulating hypotheses, and designing experiments, which in turn lead to additional observations, hypotheses, and experiments in repeated cycles (Figure \(\PageIndex{1}\)). Eventually, after the work of many scientists, those cycles might result in laws or theories.
Scientific Laws
After many experimental data have been collected and analyzed, the scientific community may begin to think that the results are sufficiently reproducible (i.e., dependable) to merit being summarized in a law, a verbal or mathematical description of a phenomenon that allows for general predictions. A law simply says what happens; it does not address the question of why. Notice that this is very different from our use of the word law in our every day lives. We might drive a certain speed because it is the law. In this sense we can consider the law to be prescriptive: we do something because the law tells us to. Natural laws are not prescriptive but descriptive. An apple does not fall from a tree because it is written on a sign somewhere. Instead, someone writes down the law of gravity because they observed that the movement of apples and other objects followed a pattern. Laws are unlikely to change greatly over time unless later laws are found which apply to contexts which were not considered at the time.
A verbal or mathematical description of a phenomenon that allows for general predictions.
One example of a law, the Law of Definite Proportions, was discovered by the French scientist Joseph Proust (1754–1826). This law states that a chemical substance always contains the same proportions of elements by mass. Thus sodium chloride (table salt) always contains the same proportion by mass of sodium to chlorine, in this case 39.34% sodium and 60.66% chlorine by mass, and sucrose (table sugar) is always 42.11% carbon, 6.48% hydrogen, and 51.41% oxygen by mass. The law does not say why the elements exist in the same proportion every time, simply that they do.
Scientific Theories
Whereas a law states only what happens, a theory explains why nature behaves as it does. How is a theory different than a hypothesis? When one makes a hypothesis, there is generally a reason to believe something about what will happen, but it is not necessarily generalizable beyond the particular situation which is being investigated. To be accepted as a scientific theory, there needs to be a preponderance of evidence to support a particular explanation for observed phenomena. Theories are not considered to be more or less valid than laws by those in the scientific community, they are simply different types of information. This is in contrast to how we use these terms in our regular lives. We often use the word theory to represent something that is at best a hypothesis, and more likely a conjecture. For example "My theory is that at the end of this mystery novel, Moriarty will be the culprit." This use of the word theory is merely conjecture about a fictional character, not a combination of explanations coming from multiple experiments performed by several research groups. So when we use the term "it was just a theory" we should be careful not to apply that to scientific theories. Theories don't "graduate" into a law when they have sufficient evidence. They are an explanation as opposed to a description. In a few cases, technological advancements have allowed us to see things that we couldn't before. For example, the atomic theory was considered a well established fact long before the first atoms were observed with electron microscopes. However, we still refer to it as the atomic theory because it has explanatory power for a few scientific laws, such as the law of definite proportions that we just mentioned.
When a preponderance of evidence supports a particular explanation for observed phenomena.
The theory developed to explain the extinction of the dinosaurs, for example, is that Earth occasionally encounters small- to medium-sized asteroids, and these encounters may have unfortunate implications for the continued existence of most species. The hypothesis that this might be the case was proposed by Luis and Walter Alvarez based on their discovery of a rare element in a geological site in Italy in the 1980s. Over the course of a few decades, this hypothesis became recognized as a theory as additional data emerged to support it. This theory is consistent with the bulk of evidence amassed to date by multiple researchers over many different disciplines. Figure \(\PageIndex{2}\) summarizes the application of the scientific method in this case.
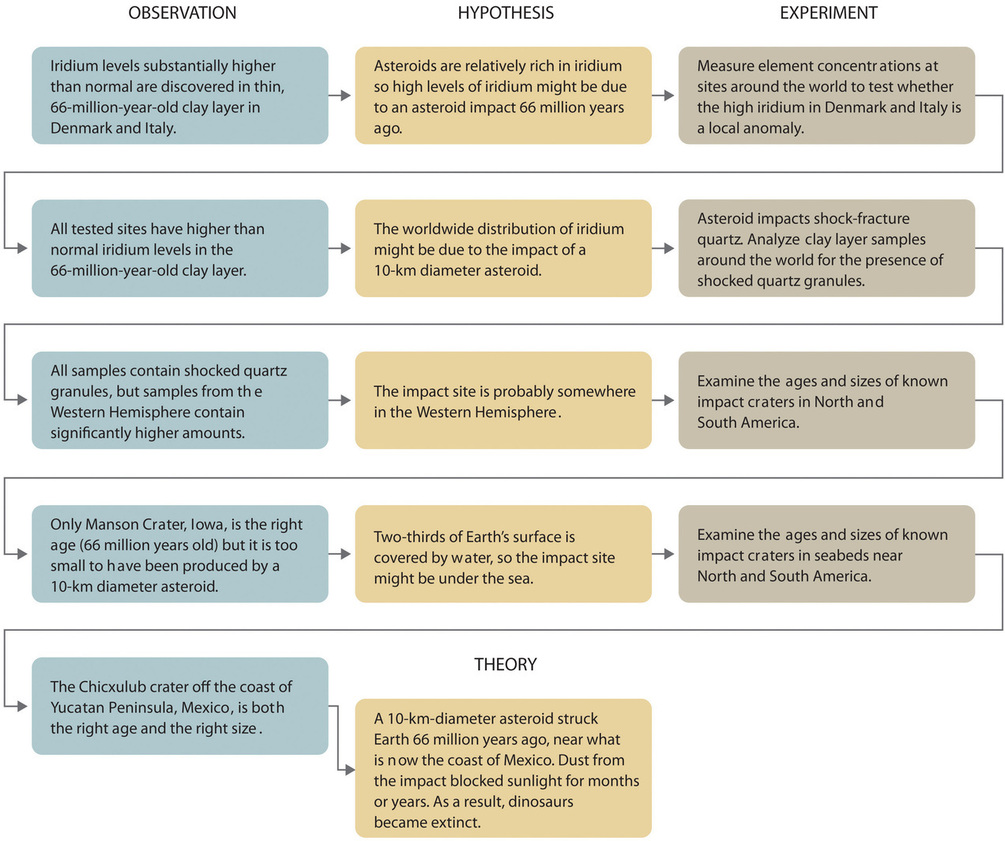
Since scientists can enter the cycle shown in Figure \(\PageIndex{1}\) at any point, the actual application of the scientific method to different topics can take many different forms, as we shall see in the next subsection.
It is important to remember that scientists have a tendency to formulate hypotheses in familiar terms simply because it is difficult to propose something that has never been encountered or imagined before. As a result, scientists sometimes discount or overlook unexpected findings that disagree with the basic assumptions behind the hypothesis or theory being tested. Fortunately, truly important findings are immediately subject to independent verification by scientists in other laboratories, which science is a self-correcting discipline. When the Alvarezes originally suggested that an extraterrestrial impact caused the extinction of the dinosaurs, the response was almost universal skepticism and scorn. In only 20 years, however, the persuasive nature of the evidence overcame the skepticism of many scientists, and their initial hypothesis has now evolved into a theory that has revolutionized paleontology and geology.
Section Summary
- Scientific laws are verbal or mathematical descriptions of a phenomenon that allow for general predictions.
- A scientific theory exists when a preponderance of evidence supports a particular explanation for observed phenomena.
Contributors
Curated from resources found in this text by Libretexts.


