10.8: Ionization Constants of Weak Acids
- Page ID
- 233061
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
| Acid | Formula | <emKa at 25 °C | Lewis Structure |
|---|---|---|---|
| acetic | CH3CO2H | 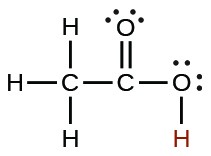 |
|
| arsenic | H3AsO4 | 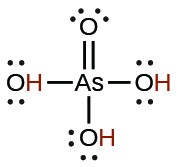 |
|
| H2AsO4− | |||
| HAsO42− | |||
| arsenous | H3AsO3 | 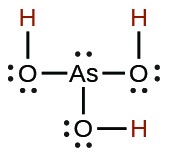 |
|
| boric | H3BO3 | 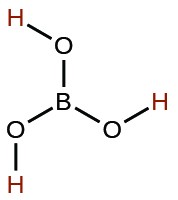 |
|
| carbonic | H2CO3 | 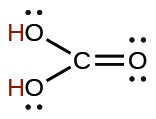 |
|
| HCO3− | |||
| cyanic | HCNO | 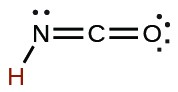 |
|
| formic | HCO2H | 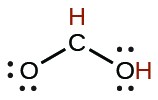 |
|
| hydrazoic | HN3 | 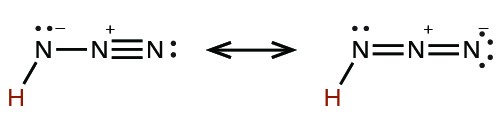 |
|
| hydrocyanic | HCN | ||
| hydrofluoric | HF | ||
| hydrogen peroxide | H2O2 |  |
|
| hydrogen selenide | H2Se | ||
| HSe– | |||
| hydrogen sulfate ion | HSO4− | 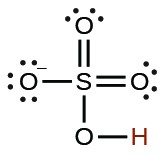 |
|
| hydrogen sulfide | H2S | ||
| HS– | |||
| hydrogen telluride | H2Te | ||
| HTe– | |||
| hypobromous | HBrO | ||
| hypochlorous | HClO | ||
| nitrous | HNO2 | 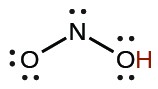 |
|
| oxyalic | H2C2O4 | 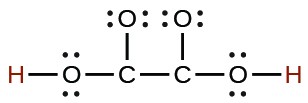 |
|
| HC2O4− | |||
| phosphoric | H3PO4 | 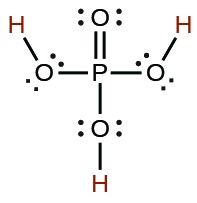 |
|
| H2PO4− | |||
| HPO42− | |||
| phosphorous | H3PO3 | 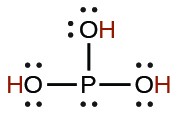 |
|
| H2PO3− | |||
| sulfurous | H2SO3 | 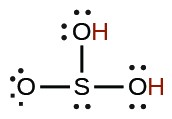 |
|
| HSO3− |
- Chemistry. Provided by: OpenStax College. Located at: http://openstaxcollege.org. License: CC BY: Attribution. License Terms: Download for free at https://openstaxcollege.org/textbooks/chemistry/get

