6.7: Formal Charges and Resonance
- Page ID
- 233027
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
By the end of this section, you will be able to:
- Compute formal charges for atoms in any Lewis structure
- Use formal charges to identify the most reasonable Lewis structure for a given molecule
- Explain the concept of resonance and draw Lewis structures representing resonance forms for a given molecule
In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable.
Calculating Formal Charge
The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure.
Thus, we calculate formal charge as follows:
We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion.
We must remember that the formal charge calculated for an atom is not the actual charge of the atom in the molecule. Formal charge is only a useful bookkeeping procedure; it does not indicate the presence of actual charges.
Example 1: Calculating Formal Charge from Lewis Structures
Assign formal charges to each atom in the interhalogen ion
[reveal-answer q=”470497″]Show Answer[/reveal-answer]
[hidden-answer a=”470497″]
- We divide the bonding electron pairs equally for all I–Cl bonds:
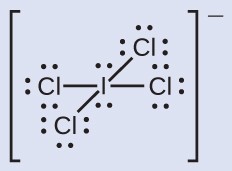
- We assign lone pairs of electrons to their atoms. Each Cl atom now has seven electrons assigned to it, and the I atom has eight.
- Subtract this number from the number of valence electrons for the neutral atom:
I: 7 – 8 = –1
Cl: 7 – 7 = 0
The sum of the formal charges of all the atoms equals –1, which is identical to the charge of the ion (–1).
[/hidden-answer]
Check Your Learning
Calculate the formal charge for each atom in the carbon monoxide molecule:

[reveal-answer q=”399385″]Show Answer[/reveal-answer]
[hidden-answer a=”399385″]C -1, O +1[/hidden-answer]
Example 2: Calculating Formal Charge from Lewis Structures
Assign formal charges to each atom in the interhalogen molecule BrCl3.
[reveal-answer q=”149263″]Show Answer[/reveal-answer]
[hidden-answer a=”149263″]
- Assign one of the electrons in each Br–Cl bond to the Br atom and one to the Cl atom in that bond:
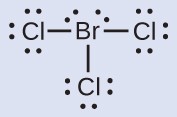
- Assign the lone pairs to their atom. Now each Cl atom has seven electrons and the Br atom has seven electrons.
- Subtract this number from the number of valence electrons for the neutral atom. This gives the formal charge:Br: 7 – 7 = 0Cl: 7 – 7 = 0All atoms in BrCl3 have a formal charge of zero, and the sum of the formal charges totals zero, as it must in a neutral molecule.
[/hidden-answer]
Check Your Learning
Determine the formal charge for each atom in NCl3.
[reveal-answer q=”185019″]Show Answer[/reveal-answer]
[hidden-answer a=”185019″]N: 0, all three Cl atoms: 0:
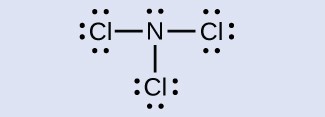
[/hidden-answer]
Using Formal Charge to Predict Molecular Structure
The arrangement of atoms in a molecule or ion is called its molecular structure. In many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure—different multiple bond and lone-pair electron placements or different arrangements of atoms, for instance. A few guidelines involving formal charge can be helpful in deciding which of the possible structures is most likely for a particular molecule or ion:
- A molecular structure in which all formal charges are zero is preferable to one in which some formal charges are not zero.
- If the Lewis structure must have nonzero formal charges, the arrangement with the smallest nonzero formal charges is preferable.
- Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign.
- When we must choose among several Lewis structures with similar distributions of formal charges, the structure with the negative formal charges on the more electronegative atoms is preferable.
To see how these guidelines apply, let us consider some possible structures for carbon dioxide, CO2. We know from our previous discussion that the less electronegative atom typically occupies the central position, but formal charges allow us to understand why this occurs. We can draw three possibilities for the structure: carbon in the center and double bonds, carbon in the center with a single and triple bond, and oxygen in the center with double bonds:
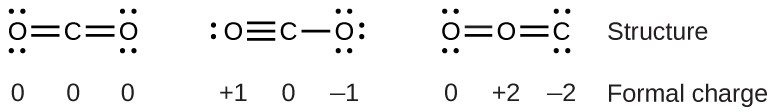
Comparing the three formal charges, we can definitively identify the structure on the left as preferable because it has only formal charges of zero (Guideline 1).
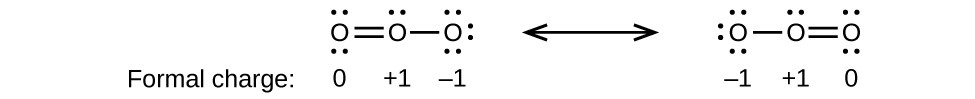
As another example, the thiocyanate ion, an ion formed from a carbon atom, a nitrogen atom, and a sulfur atom, could have three different molecular structures: CNS–, NCS–, or CSN–. The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms. Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here:
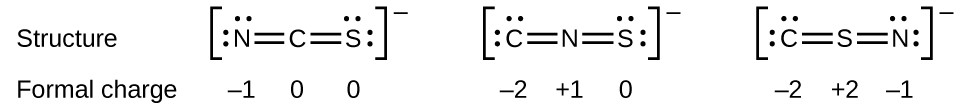
Note that the sum of the formal charges in each case is equal to the charge of the ion (–1). However, the first arrangement of atoms is preferred because it has the lowest number of atoms with nonzero formal charges (Guideline 2). Also, it places the least electronegative atom in the center, and the negative charge on the more electronegative element (Guideline 4).
Example 3: Using Formal Charge to Determine Molecular Structure
Nitrous oxide, N2O, commonly known as laughing gas, is used as an anesthetic in minor surgeries, such as the routine extraction of wisdom teeth. Which is the likely structure for nitrous oxide?

[reveal-answer q=”385530″]Show Answer[/reveal-answer]
[hidden-answer a=”385530″]
Determining formal charge yields the following:

The structure with a terminal oxygen atom best satisfies the criteria for the most stable distribution of formal charge:
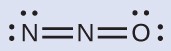
The number of atoms with formal charges are minimized (Guideline 2), and there is no formal charge larger than one (Guideline 2). This is again consistent with the preference for having the less electronegative atom in the central position.
[/hidden-answer]
Check Your Learning
Which is the most likely molecular structure for the nitrite ion?
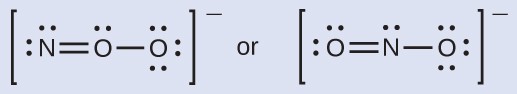
[reveal-answer q=”573302″]Show Answer[/reveal-answer]
[hidden-answer a=”573302″]ONO–[/hidden-answer]
Resonance
You may have noticed that the nitrite anion in Example 3 can have two possible structures with the atoms in the same positions. The electrons involved in the N–O double bond, however, are in different positions: 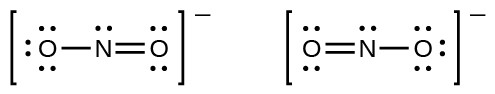
If nitrite ions do indeed contain a single and a double bond, we would expect for the two bond lengths to be different. A double bond between two atoms is shorter (and stronger) than a single bond between the same two atoms. Experiments show, however, that both N–O bonds in have the same strength and length, and are identical in all other properties.
It is not possible to write a single Lewis structure for in which nitrogen has an octet and both bonds are equivalent. Instead, we use the concept of resonance: if two or more Lewis structures with the same arrangement of atoms can be written for a molecule or ion, the actual distribution of electrons is an average of that shown by the various Lewis structures. The actual distribution of electrons in each of the nitrogen-oxygen bonds in
is the average of a double bond and a single bond. We call the individual Lewis structures resonance forms. The actual electronic structure of the molecule (the average of the resonance forms) is called a resonance hybrid of the individual resonance forms. A double-headed arrow between Lewis structures indicates that they are resonance forms. Thus, the electronic structure of the
ion is shown as:
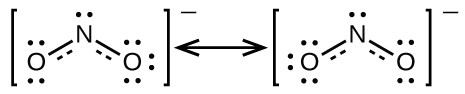
We should remember that a molecule described as a resonance hybrid never possesses an electronic structure described by either resonance form. It does not fluctuate between resonance forms; rather, the actual electronic structure is always the average of that shown by all resonance forms. George Wheland, one of the pioneers of resonance theory, used a historical analogy to describe the relationship between resonance forms and resonance hybrids. A medieval traveler, having never before seen a rhinoceros, described it as a hybrid of a dragon and a unicorn, because it had many properties in common with both. Just as a rhinoceros is neither a dragon sometimes nor a unicorn at other times, a resonance hybrid is neither of its resonance forms at any given time. Like a rhinoceros, it is a real entity that experimental evidence has shown to exist. It has some characteristics in common with its resonance forms, but the resonance forms themselves are convenient, imaginary images (like the unicorn and the dragon).
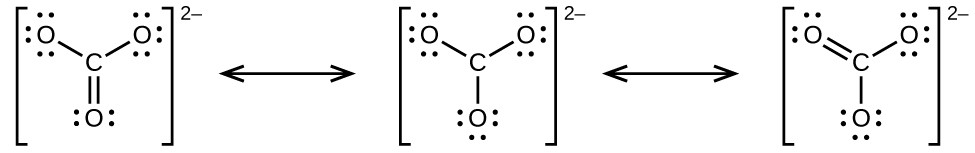
The carbonate anion, provides a second example of resonance:
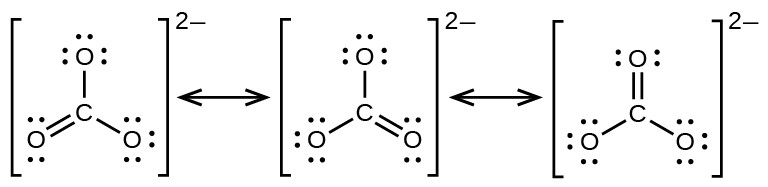
One oxygen atom must have a double bond to carbon to complete the octet on the central atom. All oxygen atoms, however, are equivalent, and the double bond could form from any one of the three atoms. This gives rise to three resonance forms of the carbonate ion. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures. Again, experiments show that all three C–O bonds are exactly the same.
Key Concepts and Summary
In a Lewis structure, formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom. These hypothetical formal charges are a guide to determining the most appropriate Lewis structure. A structure in which the formal charges are as close to zero as possible is preferred. Resonance occurs in cases where two or more Lewis structures with identical arrangements of atoms but different distributions of electrons can be written. The actual distribution of electrons (the resonance hybrid) is an average of the distribution indicated by the individual Lewis structures (the resonance forms).
Key Equations
Exercises
- Write resonance forms that describe the distribution of electrons in each of the molecules or ions given below:
- selenium dioxide, OSeO
- nitrate ion,
- nitric acid, HNO3 (N is bonded to an OH group and two O atoms)
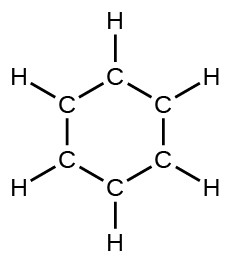
- benzene, C6H6:
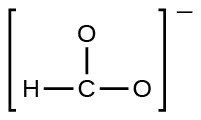
- the formate ion:
- Write resonance forms that describe the distribution of electrons in each of the molecules or ions given below:
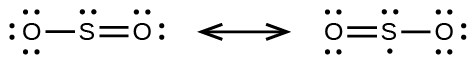
- sulfur dioxide, SO2
- carbonate ion,
- hydrogen carbonate ion,
(C is bonded to an OH group and two O atoms)
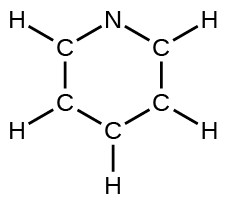
- pyridine:
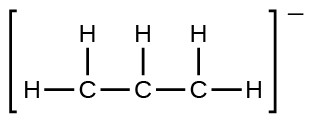
- the allyl ion:
- Write the resonance forms of ozone, O3, the component of the upper atmosphere that protects the Earth from ultraviolet radiation.
- Sodium nitrite, which has been used to preserve bacon and other meats, is an ionic compound. Write the resonance forms of the nitrite ion,
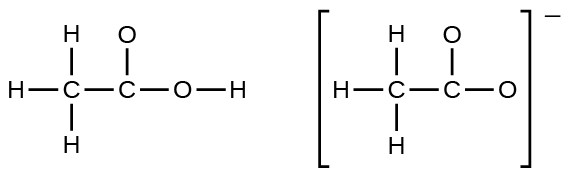
- In terms of the bonds present, explain why acetic acid, CH3CO2H, contains two distinct types of carbon-oxygen bonds, whereas the acetate ion, formed by loss of a hydrogen ion from acetic acid, only contains one type of carbon-oxygen bond. The skeleton structures of these species are shown:
- Write the Lewis structures for the following, and include resonance structures where appropriate. Indicate which of the three has the strongest carbon-oxygen bond.
- CO2
- CO
- Toothpastes containing sodium hydrogen carbonate (sodium bicarbonate) and hydrogen peroxide are widely used. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriate.
- Determine the formal charge of each element in the following:
- HCl
- CF4
- PCl3
- PF5
- Determine the formal charge of each element in the following:
- H3O+
- NH3
(e) H2O2
- Calculate the formal charge of chlorine in the molecules Cl2, BeCl2, and ClF5.
- Calculate the formal charge of each element in the following compounds and ions:
- F2CO
- NO–
- H2CCH2
- ClF3
- SeF6
- Draw all possible resonance structures for each of the compounds below. Determine the formal charge on each atom in each of the resonance structures:
- O3
- SO2
- Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in nitrosyl chloride: ClNO or ClON?
- Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in hypochlorous acid: HOCl or OClH?
- Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in sulfur dioxide: OSO or SOO?
- Draw the structure of hydroxylamine, H3NO, and assign formal charges; look up the structure. Is the actual structure consistent with the formal charges?
- Iodine forms a series of fluorides (listed below). Write Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule:
- IF
- IF3
- IF5
- IF7
- Write the Lewis structure and chemical formula of the compound with a molar mass of about 70 g/mol that contains 19.7% nitrogen and 80.3% fluorine by mass, and determine the formal charge of the atoms in this compound.
- Which of the following structures would we expect for nitrous acid? Determine the formal charges:

- Sulfuric acid is the industrial chemical produced in greatest quantity worldwide. About 90 billion pounds are produced each year in the United States alone. Write the Lewis structure for sulfuric acid, H2SO4, which has two oxygen atoms and two OH groups bonded to the sulfur.
[reveal-answer q=”793559″]Selected Answers[/reveal-answer]
[hidden-answer a=”793559″]
2. The resonance forms are as follows:
- sulfur dioxide, SO2:
- carbonate ion,
:
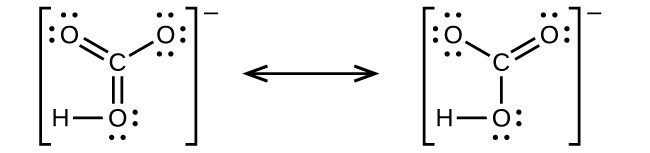
- hydrogen carbonate ion,
(C is bonded to an OH group and two O atoms):
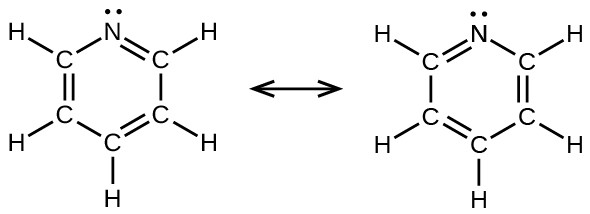
- pyridine:
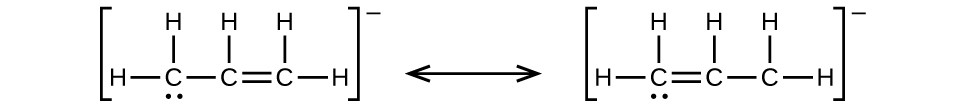
- the allyl ion:
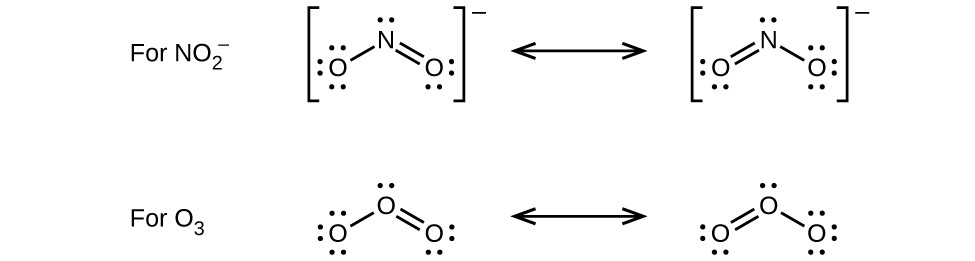
4. 
6. The Lewis structures are as follows:
- CO2:
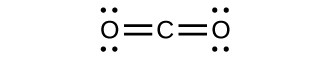
- CO:
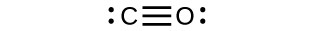
CO has the strongest carbon-oxygen bond, because there are is a triple bond joining C and O. CO2 has double bonds, and carbonate has 1.3 bonds.
8.
| Element | Bonding Electrons | Nonbonded Electrons | Valence Electrons | Formal Charge | |
|---|---|---|---|---|---|
| (a) | H Cl |
1 1 |
0 6 |
1 7 |
0 0 |
| (b) | C F |
4 1 |
0 6 |
4 7 |
0 0 |
| (c) | P Cl |
3 1 |
2 6 |
5 7 |
0 0 |
| (d) | P F |
5 1 |
0 6 |
5 7 |
0 0 |
10.
| Element | Bonding Electrons | Nonbonded Electrons | Valence Electrons | Formal Charge | |
|---|---|---|---|---|---|
| Cl2 | Cl | 1 | 6 | 7 | 0 |
| BeCl2 | Be Cl |
2 1 |
0 6 |
2 7 |
0 0 |
| ClF5 | Cl F |
5 1 |
2 6 |
7 7 |
0 0 |
12. Draw all possible resonance structures for each of the compounds below. Determine the formal charge on each atom in each of the resonance structures:
- O3:
- SO2:
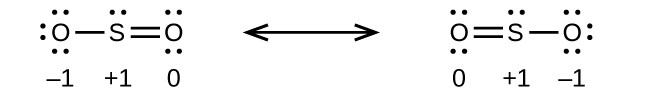
:
![[Two Lewis structures are shown, with brackets surrounding each with a superscripted negative sign and a double ended arrow in between. The left structure shows a nitrogen atom with one lone pair of electrons single bonded to an oxygen atom with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. The symbols and numbers below this structure read “open parenthesis, 0, close parenthesis, open parenthesis, 0, close parenthesis, open parenthesis, negative 1, close parenthesis. The right structure appears as a mirror image of the left and the symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis, open parenthesis, 0, close parenthesis, open parenthesis, 0, close parenthesis.]](https://chem.libretexts.org/@api/deki/files/290073/CNX_Chem_07_04_Exercis12c_img1.jpg?revision=1)
:
![[Three Lewis structures are shown, with brackets surrounding each with a superscripted negative sign and a double ended arrow in between. The left structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. The single bonded oxygen atoms are labeled, from the top of the structure and going clockwise, “open parenthesis, negative 1, close parenthesis, open parenthesis, positive 1, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, 0, close parenthesis, open parenthesis, negative 1, close parenthesis. The middle structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons, one of which is labeled “open parenthesis, positive 1, close parenthesis” and double bonded to an oxygen atom with two lone pairs of electrons labeled “open parenthesis, 0, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis, open parenthesis, negative 1, close parenthesis. The right structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. One of the single bonded oxygen atoms is labeled, “open parenthesis, negative 1, close parenthesis while the double bonded oxygen is labeled, “open parenthesis, positive 1, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis” and “open parenthesis, 0, close parenthesis”.]](https://chem.libretexts.org/@api/deki/files/290075/CNX_Chem_07_04_Exercis12d_img1.jpg?revision=1)
14. 
The structure with formal charges of 0 is the most stable and would therefore be the correct arrangement of atoms.
16. The structure that gives zero formal charges is consistent with the actual structure:
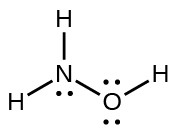
18. There are 19.7 g N and 80.3 g F in a 100.0-g sample:
The empirical formula is NF3 and its molar mass is 71.00 g/mol, which is consistent with the stated molar mass.
- Oxidation states: N = +3, F = –1.
- Formal charges: N = 0, F = 0:
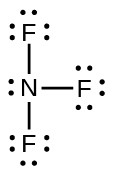
20. The Lewis structure is
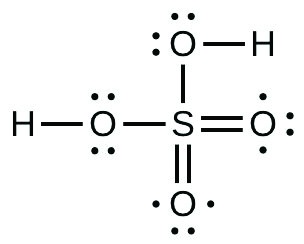
[/hidden-answer]
Glossary
formal charge: charge that would result on an atom by taking the number of valence electrons on the neutral atom and subtracting the nonbonding electrons and the number of bonds (one-half of the bonding electrons)
molecular structure: arrangement of atoms in a molecule or ion
resonance: situation in which one Lewis structure is insufficient to describe the bonding in a molecule and the average of multiple structures is observed
resonance forms: two or more Lewis structures that have the same arrangement of atoms but different arrangements of electrons
resonance hybrid: average of the resonance forms shown by the individual Lewis structures
- Chemistry. Provided by: OpenStax College. Located at: http://openstaxcollege.org. License: CC BY: Attribution. License Terms: Download for free at https://openstaxcollege.org/textbooks/chemistry/get

