3.4: Elements and Compounds
- Page ID
- 177888
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
The famous fictional British detective Sherlock Holmes was often said to make the statement "elementary, my dear Watson". In reality, the closest he ever came to that line was an exchange with Watson in the short story "The Crooked Man". Holmes shows shrewd insight into Watson's activities of the day. When asked how he knew what Watson was doing, Holmes simply replies "Elementary". Regardless of exactly how he put it, Sherlock was simply referring to what the Free Dictionary defines as "relating to, or constituting the basic, essential, or fundamental part".
Elements
An element is the simplest form of matter that has a unique set of properties. Examples of well-known elements include oxygen, iron, and gold (see below). Elements cannot be broken down into a simpler substance. Likewise, one element cannot be chemically converted into a different element.

Chemical elements are the simplest of substances. (A) An oxygen tank like this is used by people with a need for breathing assistance. (B) A simple skillet can be made from cast iron. (C) Gold bars are formed and used for monetary purposes.
Some elements have been known for centuries (gold, silver, iron, and copper among others) while others have been created in the lab only within the last several years. Most elements do not exist as such in nature. They are so reactive that they can be found only in combination with other materials.
Several of the elements are very valuable while others are quite inexpensive. Gold is currently worth almost $1700 per ounce. Aluminum, on the other hand, only sells for about 90 cents per pound, considerably lower than gold. Copper is worth somewhat more, selling for approximately $3.50 per pound. Platinum is very valuable at about $1650 an ounce, not quite as expensive as gold.
Explore More
Select five elements from this interactive Periodic Table
Answer the following questions:
1. What is the name of the element?
2. When was it discovered?
3. Who discovered it?
4. What is one use for this element?
Compounds
A compound is a substance that contains two or more elements chemically combined in a fixed proportion. The elements carbon and hydrogen combine to form many different compounds. One of the simplest is called methane, in which there are always four times as many hydrogen particles as carbon particles. Methane is a pure substance because it always has the same composition. However, it is not an element because it can be broken down into simpler substances - carbon and hydrogen.
Recall that the components of a mixture can be separated from one another by physical means. This is not true for a compound. Table salt is a compound consisting of equal parts of the elements sodium and chlorine. Salt cannot be separated into its two elements by filtering, distillation, or any other physical process. Salt and other compounds can only be decomposed into their elements by a chemical process. A chemical change is a change that produces matter with a different composition. Many compounds can be decomposed into their elements by heating. When sugar is heated, it decomposes into carbon and water. Water is still a compound, but one which cannot be broken down into hydrogen and oxygen by heating. Instead, the passage of an electrical current through water will produce hydrogen and oxygen gases.
The properties of compounds are generally very different than the properties of the elements from which the compound is formed. Sodium is an extremely reactive soft metal that cannot be exposed to air or water. Chlorine is a deadly gas. The compound sodium chloride is a white solid which is essential for all living things (see below).
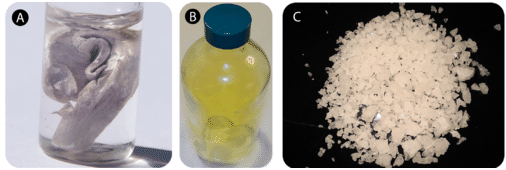
(A) Sodium is so reactive that it must be stored under oil. (B) Chlorine is a poisonous yellow-green gas. (C) Salt crystals, a compound of sodium and chlorine.
Summary
- An element is the simplest form of matter that has a unique set of properties.
- One element cannot be chemically converted to another element.
- A compound is a substance that contains two or more elements chemically combined in a fixed proportion.

