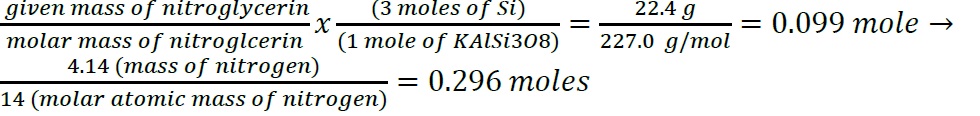3.8: Stoichiometry (Exercises)
- Last updated
- Save as PDF
- Page ID
- 148552
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)3.1: Chemical Equations
Conceptual Problems
- How does a balanced chemical equation agree with the law of definite proportions?
- What is the difference between S8 and 8S? Use this example to explain why subscripts in a formula must not be changed.
- What factors determine whether a chemical equation is balanced?
- What information can be obtained from a balanced chemical equation? Does a balanced chemical equation give information about the rate of a reaction?
Numerical Problems
1. Balance each chemical equation.
a. KI(aq) + Br2(l) → KBr(aq) + I2(s)
b. MnO2(s) + HCl(aq) → MnCl2(aq) + Cl2(g) + H2O(l)
c. Na2O(s) + H2O(l) → NaOH(aq)
d. Cu(s) + AgNO3(aq) → Cu(NO3)2(aq) + Ag(s)
e. SO2(g) + H2O(l) → H2SO3(aq)
f. S2Cl2(l) + NH3(l) → S4N4(s) + S8(s) + NH4Cl(s)
2. Balance each chemical equation.
a. Be(s) + O2(g) → BeO(s)
b. N2O3(g) + H2O(l) → HNO2(aq)
c. Na(s) + H2O(l) → NaOH(aq) + H2(g)
d. CaO(s) + HCl(aq) → CaCl2(aq) + H2O(l)
e. CH3NH2(g) + O2(g) → H2O(g) + CO2(g) + N2(g)
f. Fe(s) + H2SO4(aq) → FeSO4(aq) + H2(g)
3. Balance each chemical equation.
a. N2O5(g) → NO2(g) + O2(g)
b. NaNO3(s) → NaNO2(s) + O2(g)
c. Al(s) + NH4NO3(s) → N2(g) + H2O(l) + Al2O3(s)
d. C3H5N3O9(l) → CO2(g) + N2(g) + H2O(g) + O2(g)
e. reaction of butane with excess oxygen
f. IO2F(s) + BrF3(l) → IF5(l) + Br2(l) + O2(g)
4. Balance each chemical equation.
a. H2S(g) + O2(g) → H2O(l) + S8(s)
b. KCl(aq) + HNO3(aq) + O2(g) → KNO3(aq) + Cl2(g) + H2O(l)
c. NH3(g) + O2(g) → NO(g) + H2O(g)
d. CH4(g) + O2(g) → CO(g) + H2(g)
e. NaF(aq) + Th(NO3)4(aq) → NaNO3(aq) + ThF4(s)
f. Ca5(PO4)3F(s) + H2SO4(aq) + H2O(l) → H3PO4(aq) + CaSO4•2H2O(s) + HF(aq)
5. Balance each chemical equation.
a. NaCl(aq) + H2SO4(aq) → Na2SO4(aq) + HCl(g)
b. K(s) + H2O(l) → KOH(aq) + H2(g)
c. reaction of octane with excess oxygen
d. S8(s) + Cl2(g) → S2Cl2(l)
e. CH3OH(l) + I2(s) + P4(s) → CH3I(l) + H3PO4(aq) + H2O(l)
f. (CH3)3Al(s) + H2O(l) → CH4(g) + Al(OH)3(s)
6. Write a balanced chemical equation for each reaction.
a. Aluminum reacts with bromine.
b. Sodium reacts with chlorine.
c. Aluminum hydroxide and acetic acid react to produce aluminum acetate and water.
d. Ammonia and oxygen react to produce nitrogen monoxide and water.
e. Nitrogen and hydrogen react at elevated temperature and pressure to produce ammonia.
f. An aqueous solution of barium chloride reacts with a solution of sodium sulfate.
7. Write a balanced chemical equation for each reaction.
a. Magnesium burns in oxygen.
b. Carbon dioxide and sodium oxide react to produce sodium carbonate.
c. Aluminum reacts with hydrochloric acid.
d. An aqueous solution of silver nitrate reacts with a solution of potassium chloride.
e. Methane burns in oxygen.
f. Sodium nitrate and sulfuric acid react to produce sodium sulfate and nitric acid.
3.3: Formula Masses
Conceptual Problems
- What is the relationship between an empirical formula and a molecular formula
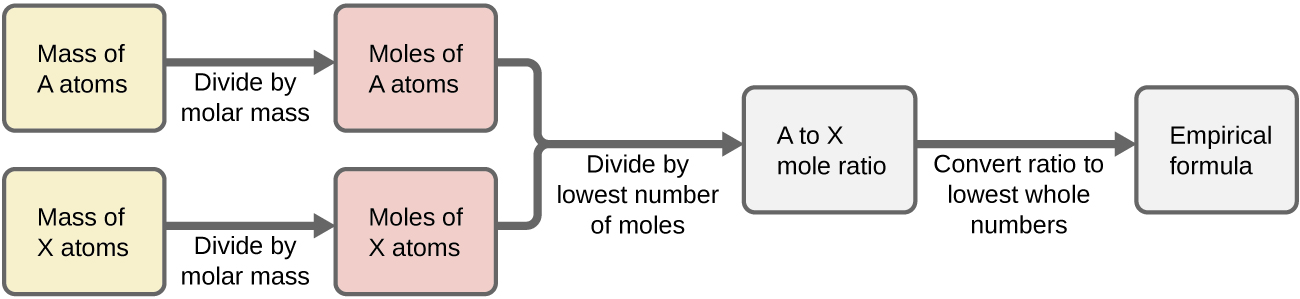
- Construct a flowchart showing how you would determine the empirical formula of a compound from its percent composition.
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 2 (Section 3.7 "Essential Skills 2") before proceeding to the Numerical Problems.
a. What is the formula mass of each species?
a. ammonium chloride
b. sodium cyanide
c. magnesium hydroxide
d. calcium phosphate
e. lithium carbonate
f. hydrogen sulfite ion
b. What is the molecular or formula mass of each compound?
a. potassium permanganate
b. sodium sulfate
c. hydrogen cyanide
d. potassium thiocyanate
e. ammonium oxalate
f. lithium acetate
1. What is the mass percentage of water in each hydrate?
a. H3AsO4·5H2O
b. NH4NiCl3·6H2O
c. Al(NO3)3·9H2O
2. What is the mass percentage of water in each hydrate?
a. CaSO4·2H2O
b. Fe(NO3)3·9H2O
c. (NH4)3ZrOH(CO3)3·2H2O
3. Which of the following has the greatest mass percentage of oxygen—KMnO4, K2Cr2O7, or Fe2O3?
4. Which of the following has the greatest mass percentage of oxygen—ThOCl2, MgCO3, or NO2Cl?
5. Calculate the percent composition of the element shown in bold in each compound.
a. SbBr3
b. As2I4
c. AlPO4
d. C6H10O
6. Calculate the percent composition of the element shown in bold in each compound.
a. HBrO3
b CsReO4
c. C3H8O
d. FeSO4
7. A sample of a chromium compound has a molar mass of 151.99 g/mol. Elemental analysis of the compound shows that it contains 68.43% chromium and 31.57% oxygen. What is the identity of the compound?
8. The percentages of iron and oxygen in the three most common binary compounds of iron and oxygen are given in the following table. Write the empirical formulas of these three compounds.
| Compound | % Iron | % Oxygen | Empirical Formula |
|---|---|---|---|
| 1 | 69.9 | 30.1 | |
| 2 | 77.7 | 22.3 | |
| 3 | 72.4 | 27.6 |
9. What is the mass percentage of water in each hydrate?
a. LiCl·H2O
b. MgSO4·7H2O
c. Sr(NO3)2·4H2O
10. What is the mass percentage of water in each hydrate?
a. CaHPO4·2H2O
b. FeCl2·4H2O
c. Mg(NO3)2·4H2O
11. Two hydrates were weighed, heated to drive off the waters of hydration, and then cooled. The residues were then reweighed. Based on the following results, what are the formulas of the hydrates?
| Compound | Initial Mass (g) | Mass after Cooling (g) |
|---|---|---|
| NiSO4·xH2O | 2.08 | 1.22 |
| CoCl2·xH2O | 1.62 | 0.88 |
12. Which contains the greatest mass percentage of sulfur—FeS2, Na2S2O4, or Na2S?
13. Given equal masses of each, which contains the greatest mass percentage of sulfur—NaHSO4 or K2SO4?
14. Calculate the mass percentage of oxygen in each polyatomic ion.
a. bicarbonate
b. chromate
c. acetate
d. sulfite
15. Calculate the mass percentage of oxygen in each polyatomic ion.
a. oxalate
b. nitrite
c. dihydrogen phosphate
d. thiocyanate
16. The empirical formula of garnet, a gemstone, is Fe3Al2Si3O12. An analysis of a sample of garnet gave a value of 13.8% for the mass percentage of silicon. Is this consistent with the empirical formula?
17. A compound has the empirical formula C2H4O, and its formula mass is 88 g. What is its molecular formula?
18. Mirex is an insecticide that contains 22.01% carbon and 77.99% chlorine. It has a molecular mass of 545.59 g. What is its empirical formula? What is its molecular formula?
19. How many moles of CO2 and H2O will be produced by combustion analysis of 0.010 mol of styrene?
20. How many moles of CO2, H2O, and N2 will be produced by combustion analysis of 0.0080 mol of aniline?
21. How many moles of CO2, H2O, and N2 will be produced by combustion analysis of 0.0074 mol of aspartame?
22. How many moles of CO2, H2O, N2, and SO2 will be produced by combustion analysis of 0.0060 mol of penicillin G?
23. Combustion of a 34.8 mg sample of benzaldehyde, which contains only carbon, hydrogen, and oxygen, produced 101 mg of CO2 and 17.7 mg of H2O.
a. What was the mass of carbon and hydrogen in the sample?
b. Assuming that the original sample contained only carbon, hydrogen, and oxygen, what was the mass of oxygen in the sample?
c. What was the mass percentage of oxygen in the sample?
d. What is the empirical formula of benzaldehyde?
e. The molar mass of benzaldehyde is 106.12 g/mol. What is its molecular formula?
24. Salicylic acid is used to make aspirin. It contains only carbon, oxygen, and hydrogen. Combustion of a 43.5 mg sample of this compound produced 97.1 mg of CO2 and 17.0 mg of H2O.
a. What is the mass of oxygen in the sample?
b. What is the mass percentage of oxygen in the sample?
c. What is the empirical formula of salicylic acid?
d. The molar mass of salicylic acid is 138.12 g/mol. What is its molecular formula?
25. Given equal masses of the following acids, which contains the greatest amount of hydrogen that can dissociate to form H+—nitric acid, hydroiodic acid, hydrocyanic acid, or chloric acid?
26. Calculate the formula mass or the molecular mass of each compound.
a. heptanoic acid (a seven-carbon carboxylic acid)
b. 2-propanol (a three-carbon alcohol)
c. KMnO4
d. tetraethyllead
e. sulfurous acid
f. ethylbenzene (an eight-carbon aromatic hydrocarbon)
27. Calculate the formula mass or the molecular mass of each compound.
a. MoCl5
b. B2O3
c. bromobenzene
d. cyclohexene
e. phosphoric acid
f. ethylamine
28. Given equal masses of butane, cyclobutane, and propene, which contains the greatest mass of carbon?
29. Given equal masses of urea [(NH2)2CO] and ammonium sulfate, which contains the most nitrogen for use as a fertilizer?
Conceptual Answers
1) What is the relationship between an empirical formula and a molecular formula
- An empirical formula refers to the simplest ratio of elements that is obtained from a chemical formula while a molecular formula is calculated to show the actual formula of a molecular compound.
2) Construct a flowchart showing how you would determine the empirical formula of a compound from its percent composition.
Numerical Answers
a. What is the formula mass of each species?
a. 53.49146 amu
b. 49.0072 amu
c. 58.3197 amu
d. 310.177 amu
e. 73.891 amu
f. 81.07 amu
b. What is the molecular or formula mass of each compound?
a.158.034 amu
b. 142.04 amu
c. 27.0253 amu
d. 97.181 amu
e. 124.1 amu
f. 65.99 amu
1. To two decimal places, the percentages are:
a. 5.97%
b. 37.12%
c. 43.22%
2. To two decimal places, the percentages of water are:
a. 20.93%
b. 40.13%
c. 9.52%
3. % oxygen: KMnO4, 40.50%; K2Cr2O7, 38.07%; Fe2O3, 30.06%
4. % oxygen: ThOCl2, 5.02%; MgCO3, 56.93%; NO2Cl, 39.28%
5. To two decimal places, the percentages are:
a. 66.32% Br
b. 22.79% As
c. 25.40% P
d. 73.43% C
6.
a. 61.98% Br
b. 34.69% Cs
c. 59.96% C
d. 21.11% S
7. Cr2O3.
8. Empirical Formulas
- Fe2O3
- Fe4O4
- Fe6O8
9. To two decimal places, the percentages are:
a. 29.82%
b. 51.16%
c. 25.40%
10. What is the mass percentage of water in each hydrate?
a. 20.94%
b. 36.25%
c. 32.70%
11. NiSO4 · 6H2O and CoCl2 · 6H2O
12. FeS2
13. NaHSO4
14. Calculate the mass percentage of oxygen in each polyatomic ion.
a. 78.66%
b. 55.17%
c. 54.19%
d. 59.95%
15.
a. 72.71%
b. 69.55%
c. 65.99%
d. 0%
16. The empirical formula of garnet, a gemstone, is Fe3Al2Si3O12. An analysis of a sample of garnet gave a value of 13.8% for the mass percentage of silicon. Is this consistent with the empirical formula?
No, the calculated mass percentage of silicon in garnet is 16.93%
17. C4H8O2
18.
Empirical Formula: C10Cl12
Molecular Formula: C10Cl12
19. How many moles of CO2 and H2O will be produced by combustion analysis of 0.010 mol of styrene?
Moles of CO2: 0.08 mol CO2
Moles of H2O: 0.04 mol H2O
20. How many moles of CO2, H2O, and N2 will be produced by combustion analysis of 0.0080 mol of aniline?
Mole of CO2: 0.048 mol CO2
Mole of H2O: 0.028 mol H2O
Mole of N2: 0.004 mol N2
21. How many moles of CO2, H2O, and N2 will be produced by combustion analysis of 0.0074 mol of aspartame?
Mole of CO2: 0.104 mol CO2
Mole of H2O: 0.666 mol H2O
Mole of N2: 0.0074 mol N2
22. How many moles of CO2, H2O, N2, and SO2 will be produced by combustion analysis of 0.0060 mol of penicillin G?
Mole of CO2: 0.096 mol CO2
Mole of H2O: 0.054 mol H2O
Mole of N2: 0.060 mol N2
Mole of SO2: 0.060 mol SO2
23.
a. 27.6 mg C and 1.98 mg H
b. 5.2 mg O
c. 15%
d. C7H6O
e. C7H6O
24. Salicylic acid is used to make aspirin. It contains only carbon, oxygen, and hydrogen. Combustion of a 43.5 mg sample of this compound produced 97.1 mg of CO2 and 17.0 mg of H2O.
a. What is the mass of oxygen in the sample?
70.4mg
b. What is the mass percentage of oxygen in the sample?
61.70%
c. What is the empirical formula of salicylic acid?
C7H6O3
d. The molar mass of salicylic acid is 138.12 g/mol. What is its molecular formula?
C7H6O3
25. hydrocyanic acid, HCN
26. Calculate the formula mass or the molecular mass of each compound.
a. 130.1849 amu
b. 60.1 amu
c. 158.034 amu
d. 323.4 amu
e. 82.07 amu
f. 106.17 amu
27. To two decimal places, the values are:
a. 273.23 amu
b. 69.62 amu
c. 157.01 amu
d. 82.14 amu
e. 98.00 amu
f. 45.08 amu
28. Cyclobutene
29. Urea
3.4: Avogadro's Number and the Mole
Conceptual Problems
Please be sure you are familiar with the topics discussed in Essential Skills 2 before proceeding to the Conceptual Problems.
- Describe the relationship between an atomic mass unit and a gram.
- Is it correct to say that ethanol has a formula mass of 46? Why or why not?
- If 2 mol of sodium reacts completely with 1 mol of chlorine to produce sodium chloride, does this mean that 2 g of sodium reacts completely with 1 g of chlorine to give the same product? Explain your answer.
- Construct a flowchart to show how you would calculate the number of moles of silicon in a 37.0 g sample of orthoclase (KAlSi3O8), a mineral used in the manufacture of porcelain.
- Construct a flowchart to show how you would calculate the number of moles of nitrogen in a 22.4 g sample of nitroglycerin that contains 18.5% nitrogen by mass.
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 2 before proceeding to the Numerical Problems.
1. Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular mass.
2. Calculate the molecular mass or formula mass of each compound.
- KCl (potassium chloride)
- NaCN (sodium cyanide)
- H2S (hydrogen sulfide)
- NaN3 (sodium azide)
- H2CO3 (carbonic acid)
- K2O (potassium oxide)
- Al(NO3)3 (aluminum nitrate)
- Cu(ClO4)2 [copper(II) perchlorate]
3. Calculate the molecular mass or formula mass of each compound.
- V2O4 (vanadium(IV) oxide)
- CaSiO3 (calcium silicate)
- BiOCl (bismuth oxychloride)
- CH3COOH (acetic acid)
- Ag2SO4 (silver sulfate)
- Na2CO3 (sodium carbonate)
- (CH3)2CHOH (isopropyl alcohol)
4. Calculate the molar mass of each compound.
a.
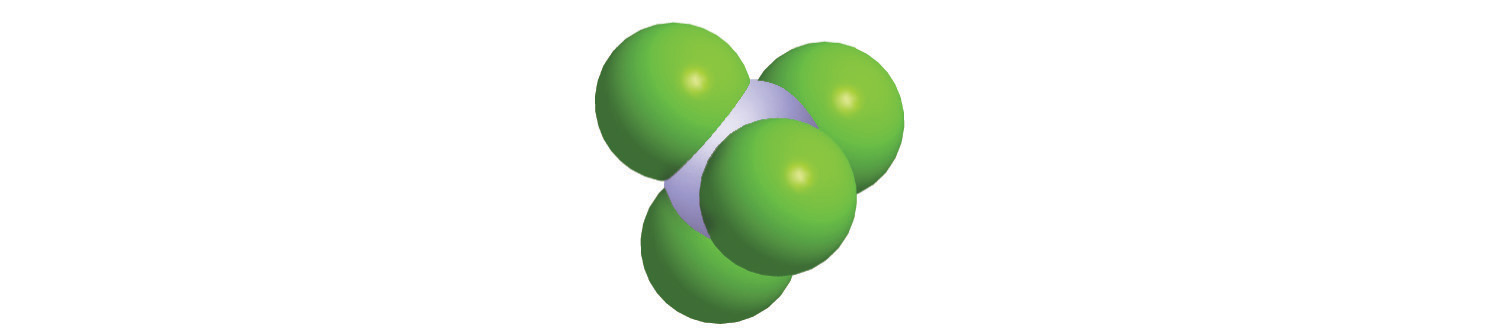
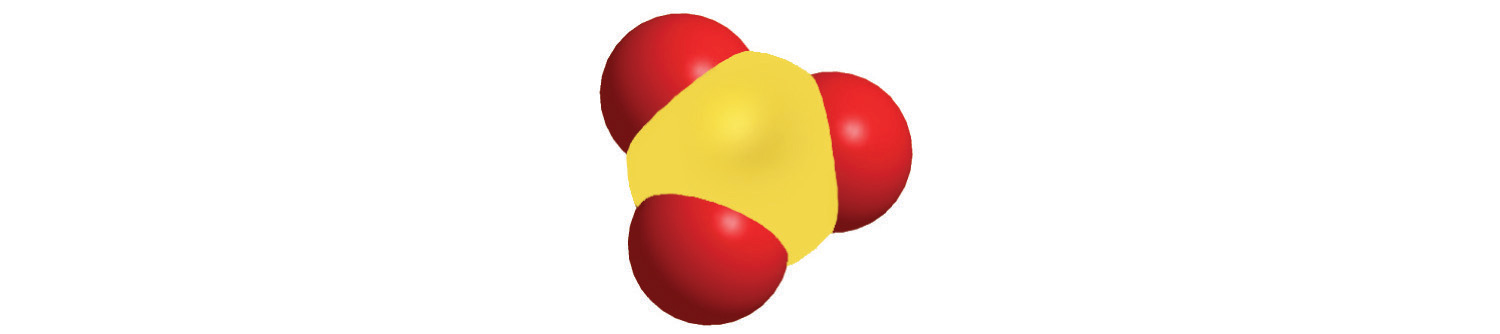
c.
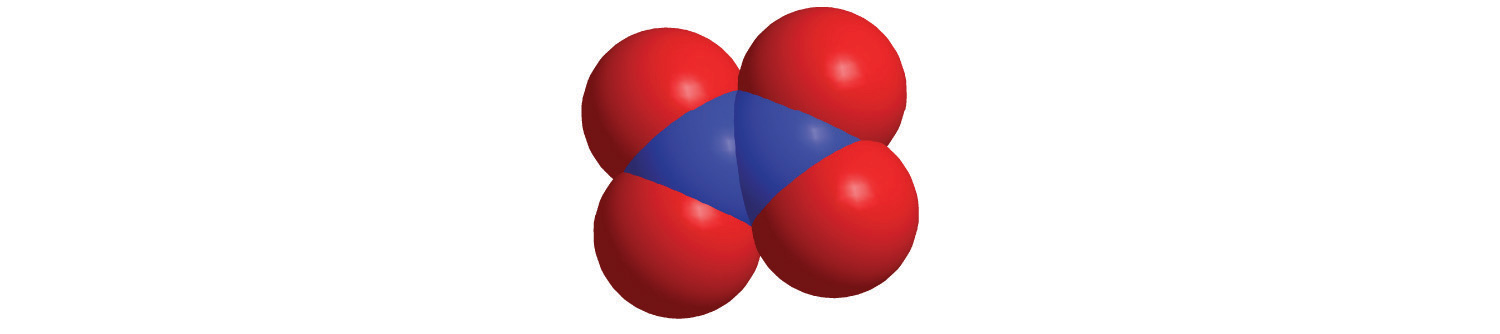
d.
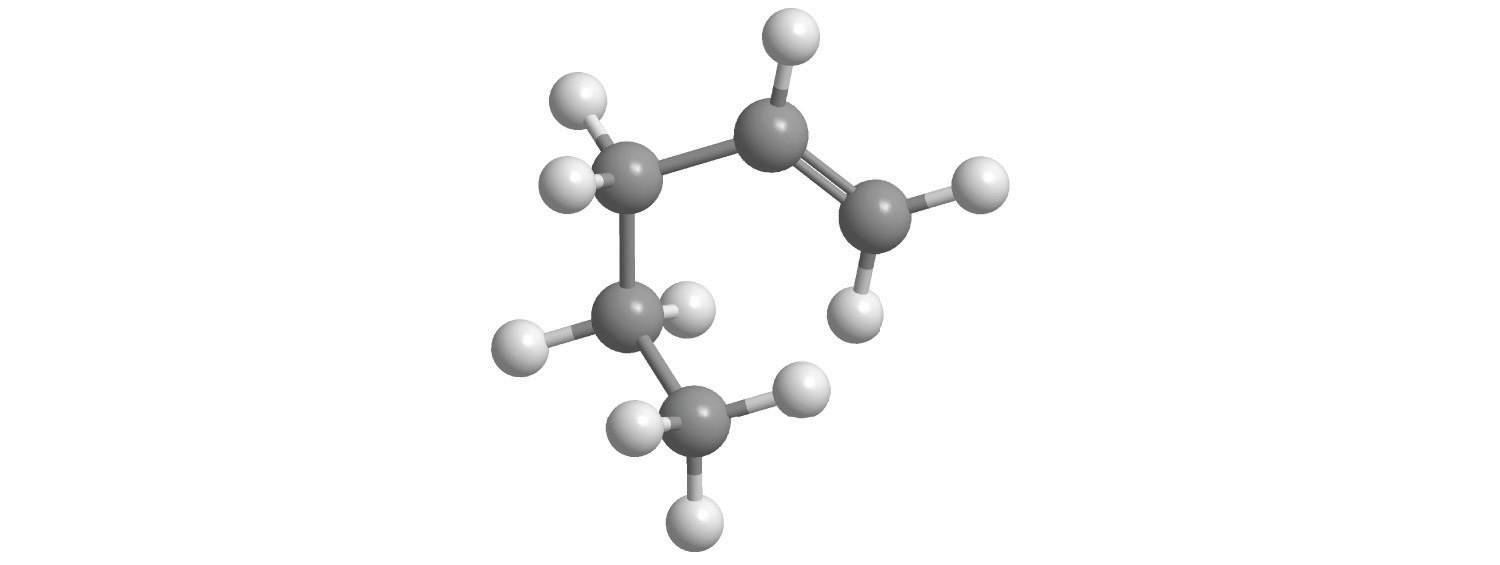
e.
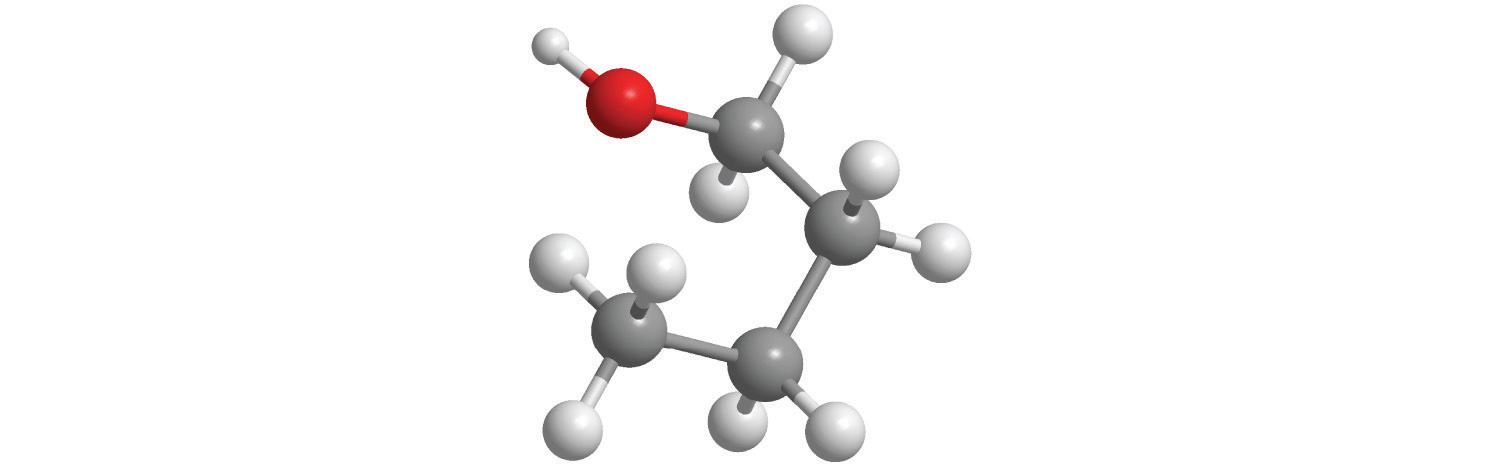
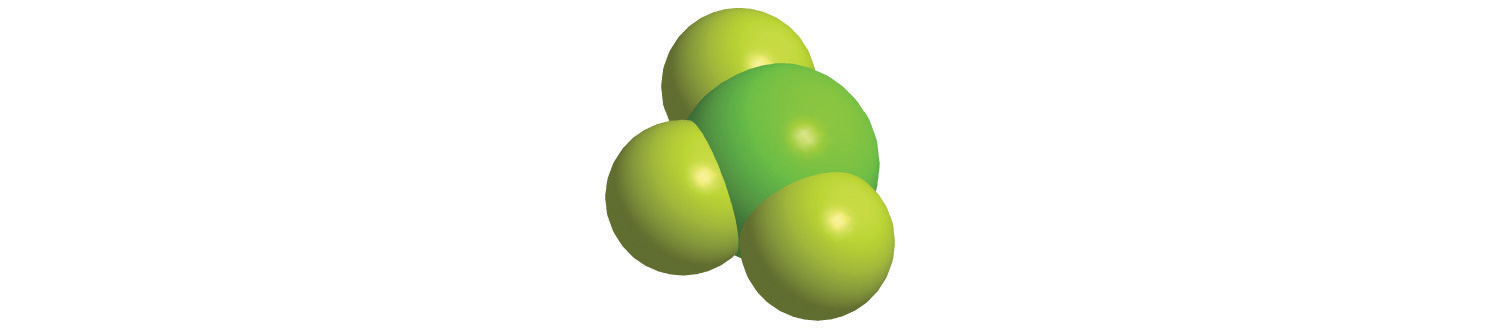
5. Calculate the molar mass of each compound.
a.
b.
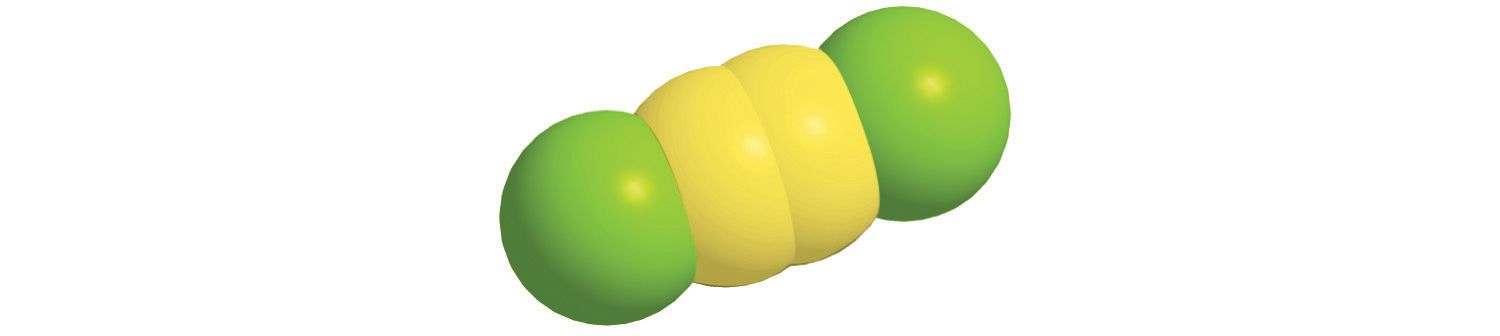
c.
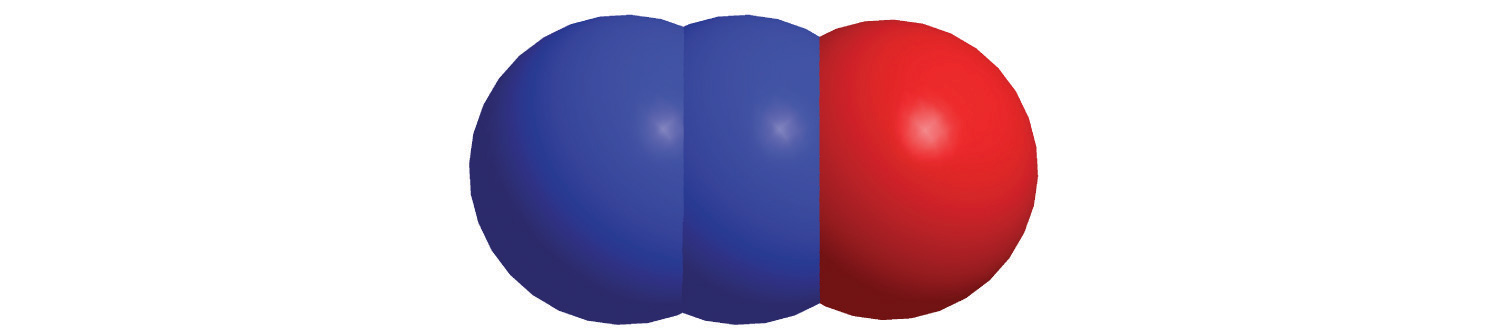
d.
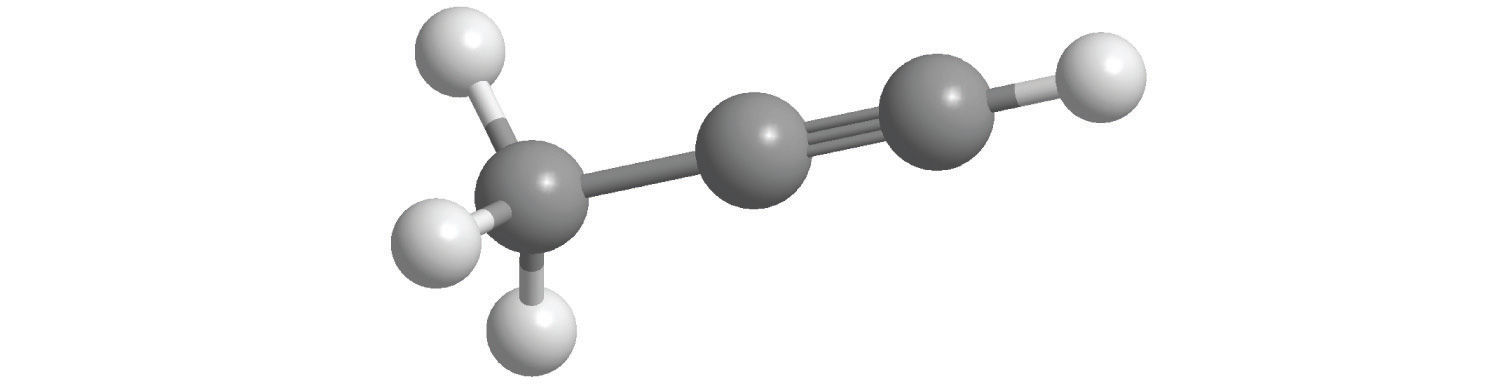
6. For each compound, write the condensed formula, name the compound, and give its molar mass.
a.
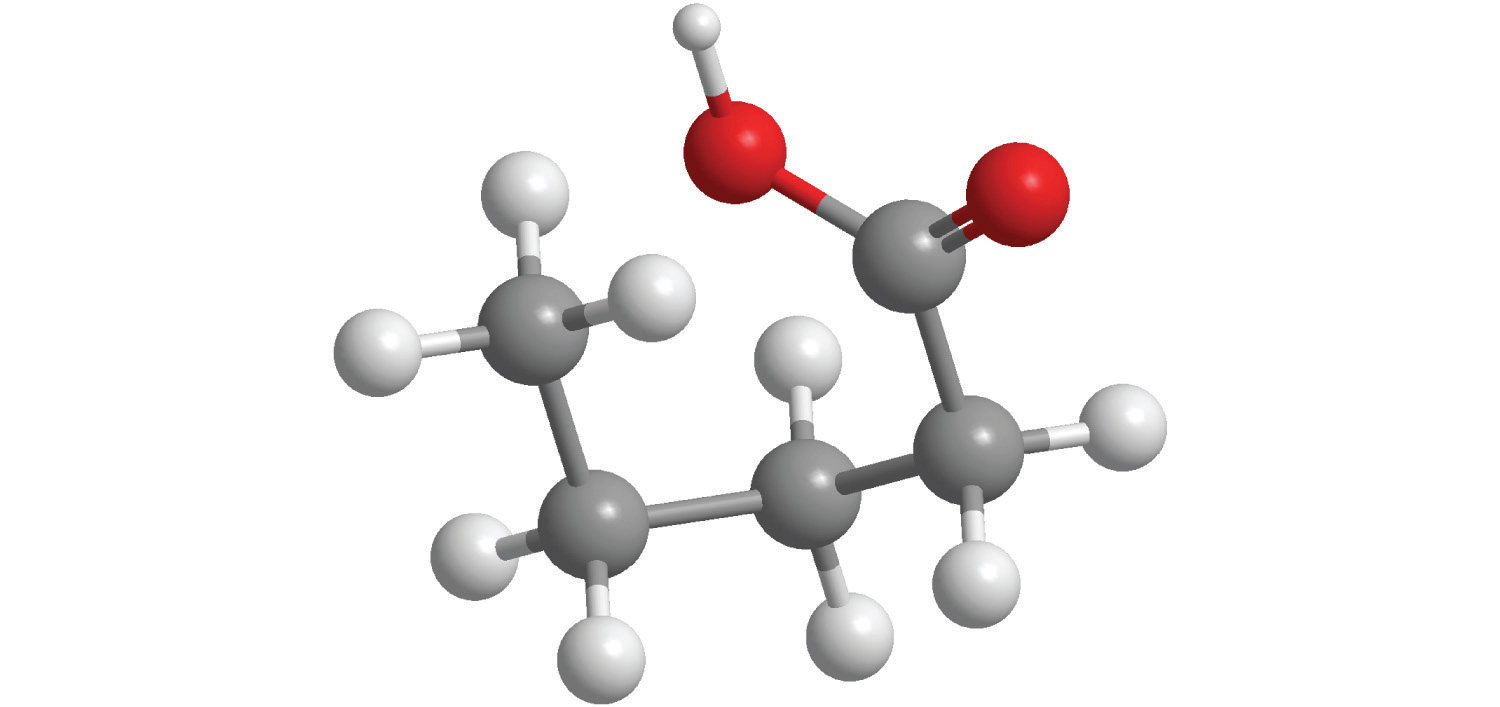
b.
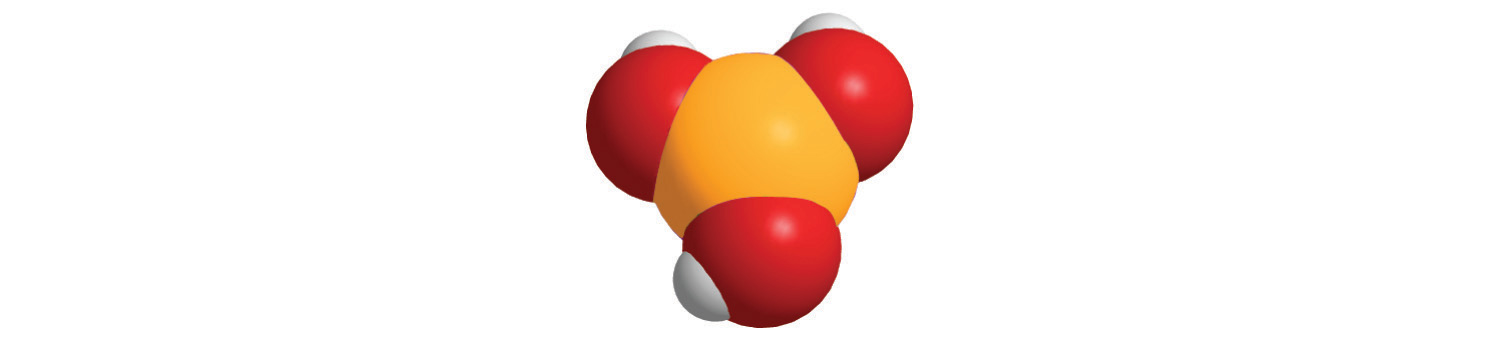
7. For each compound, write the condensed formula, name the compound, and give its molar mass.
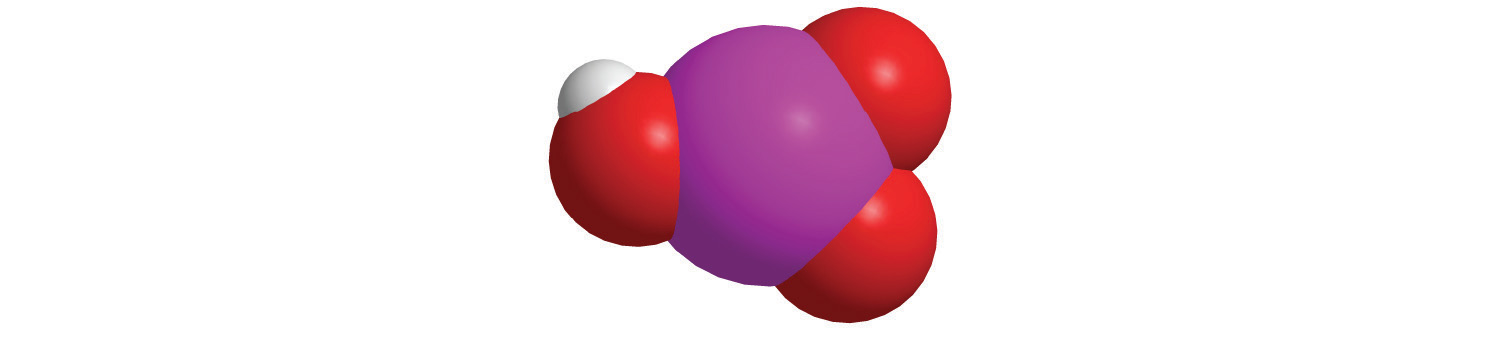
a.
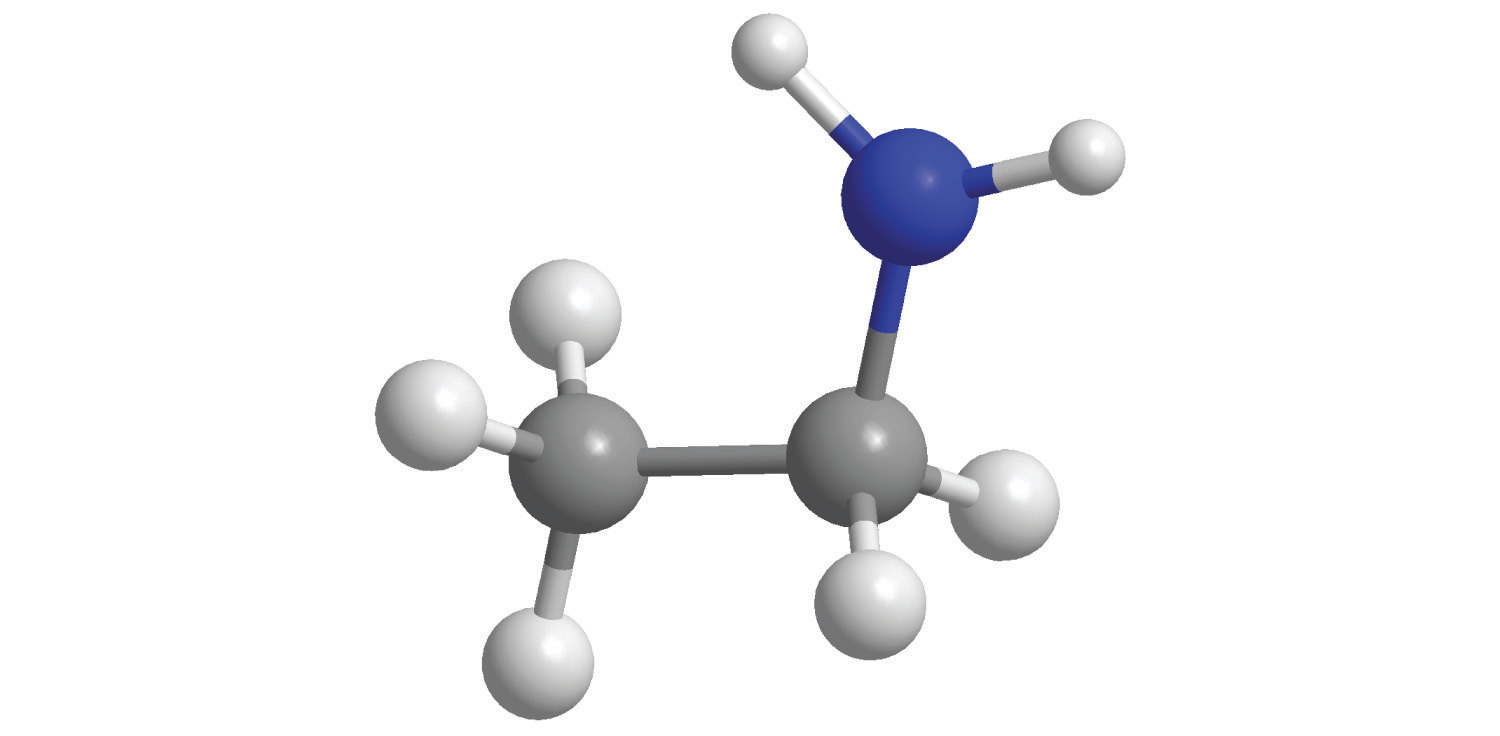
b.
8. Calculate the number of moles in 5.00 × 102 g of each substance. How many molecules or formula units are present in each sample?
a. CaO (lime)
b. CaCO3(chalk)
c. C12H22O11 [sucrose (cane sugar)]
d. NaOCl (bleach)
e. CO2 (dry ice)
9. Calculate the mass in grams of each sample.
a. 0.520 mol of N2O4
b. 1.63 mol of C6H4Br2
c. 4.62 mol of (NH4)2SO3
10. Give the number of molecules or formula units in each sample.
a. 1.30 × 10−2 mol of SCl2
b. 1.03 mol of N2O5
c. 0.265 mol of Ag2Cr2O7
11. Give the number of moles in each sample.
a. 9.58 × 1026 molecules of Cl2
b. 3.62 × 1027 formula units of KCl
c. 6.94 × 1028 formula units of Fe(OH)2
12. Solutions of iodine are used as antiseptics and disinfectants. How many iodine atoms correspond to 11.0 g of molecular iodine (I2)?
13. What is the total number of atoms in each sample?
a. 0.431 mol of Li
b. 2.783 mol of methanol (CH3OH)
c. 0.0361 mol of CoCO3
d. 1.002 mol of SeBr2O
14. What is the total number of atoms in each sample?
a. 0.980 mol of Na
b. 2.35 mol of O2
c. 1.83 mol of Ag2S
d. 1.23 mol of propane (C3H8)
15. What is the total number of atoms in each sample?
a. 2.48 g of HBr
b. 4.77 g of CS2
c. 1.89 g of NaOH
d. 1.46 g of SrC2O4
16. Decide whether each statement is true or false and explain your reasoning.
- There are more molecules in 0.5 mol of Cl2than in 0.5 mol of H2.
- One mole of H2 has 6.022 × 1023 hydrogen atoms.
- The molecular mass of H2O is 18.0 amu.
- The formula mass of benzene is 78 amu.
17. Complete the following table.
| Substance | Mass (g) | Number of Moles | Number of Molecules or Formula Units | Number of Atoms or Ions |
|---|---|---|---|---|
| MgCl2 | 37.62 | a. | b. | c. |
| AgNO3 | d. | 2.84 | e. | f. |
| BH4Cl | g. | h. | 8.93 × 1025 | i. |
| K2S | j | k. | l. | 7.69 × 1026 |
| H2SO4 | m. | 1.29 | n. | o. |
| C6H14 | 11.84 | p. | q. | r. |
| HClO3 | s. | t. | 2.45 × 1026 | u. |
18. Give the formula mass or the molecular mass of each substance.
- \(PbClF\)
- \(Cu_2P_2O_7\)
- \(BiONO_3\)
- \(Tl_2SeO_4\)
19. Give the formula mass or the molecular mass of each substance.
- \(MoCl_5\)
- \(B_2O_3\)
- \(UO_2CO_3\)
- \(NH_4UO_2AsO_4\)
Conceptual Answers
- While both are units of mass, a gram is Avogadro’s number of atomic mass units so you would multiply the number of amu by 6.022x10^23 to find total number of grams
- The correct way to state formula mass of ethanol is to show the units of mass which is amu.
- No because moles and weight operate on different set of standards meaning that they’re not equal to each other. This means that moles of different compounds contain different weights. For example, 2 moles of Na = 2 x 22.989 g = 45.98g while 1 mole of Cl = 1 x 35.453 g = 35.453 g Cl. This makes the sodium react completely with chlorine. 2g of sodium would react with = (35.453/45.978) x 2 = 1.542 g Cl
- Construct a flowchart to show how you would calculate the number of moles of silicon in a 37.0 g sample of orthoclase (KAlSi3O8), a mineral used in the manufacture of porcelain
- .
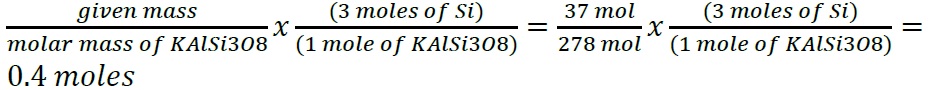
- .
- Construct a flowchart to show how you would calculate the number of moles of nitrogen in a 22.4 g sample of nitroglycerin that contains 18.5% nitrogen by mass.
- The information required to determine the mass of the solute would be the molarity of the solution because once that is achieved, volume of the solution and molar mass of the solute can be used to calculate the total mass. A derivatization that achieves this goes as: Molarity = moles of solute / volume of solution in liter -> Moles = molarity x volume in liter -> Mass= moles x molar mass.
Numerical Answers
1. Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular mass.
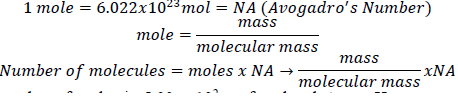
2. Calculate the molecular mass or formula mass of each compound.
- 74.55 amu
- 49.01 amu
- 34.08 amu
- 65.01 amu
- 62.02 amu
- 94.20 amu
- 213.00 amu
- 262.45 amu
3. Calculate the molecular mass or formula mass of each compound.
- 165.88 amu
- 116.16 amu
- 260.43 amu
- 60.05 amu
- 311.80 amu
- 105.99 amu
- 60.10 amu
4. Calculate the molar mass of each compound.
a. 153.82 g/mol
b. 80.06 g/mol
c. 92.01 g/mol
d. 70.13 g/mol
e. 74.12 g/mol
5. Calculate the molar mass of each compound.
a. 92.45 g/mol
b. 135.04 g/mol
c. 44.01 g/mol
d. 40.06 g/mol
6. For each compound, write the condensed formula, name the compound, and give its molar mass.
a. C5H10O2, Valeric Acid, 102.13 g/mol
b. H3PO3, Phosphorous acid, 82 g/mol
7. For each compound, write the condensed formula, name the compound, and give its molar mass.
a. C2H5NH2, Ethylamine, 45.08 g/mol
b. HIO3, Iodic acid, 175.91 g/mol
8. Calculate the number of moles in 5.00 × 102 g of each substance. How many molecules or formula units are present in each sample?
a. 5.37 × 1024 mol
b. 3.01 × 1024 mol
c. 8.80 × 1023 mol
d. 4.04 × 1024 mol
e. 6.84 × 1024 mol
9. Calculate the mass in grams of each sample.
a. 47.85 grams
b. 384.52 grams
c. 536.57 grams
10. Give the number of molecules or formula units in each sample.
a. 7.83x1021 molecules
b. 6.20x1023 molecules
c. 1.60x1023 molecules
11. Give the number of moles in each sample.
a. 1590.8 moles
b. 6011.3 moles
c. 115244.1 moles
12. Solutions of iodine are used as antiseptics and disinfectants. How many iodine atoms correspond to 11.0 g of molecular iodine (I2)?
2.61 x1022 molecules
13. What is the total number of atoms in each sample?
a. 2.60x1023 atoms
b. 1.01x1025 atoms
c. 1.09x1023 atoms
d. 2.41x1024 atoms
14. What is the total number of atoms in each sample?
a. 5.9x1023 atoms
b. 2.8x1024 atoms
c. 3.31x1024 atoms
d. 8.15x1024 atoms
15. What is the total number of atoms in each sample?
a. 3.69x1022 atoms
b. 1.13x1023 atoms
c. 8.54x1022 atoms
d. 3.50x1023 atoms
16.
a. False, the number of molecules in 0.5 mol Cl2 are the same amount of molecules in H2
b. False, the number of molecules in H2 is 2 x (6.022 x10^23) H atoms
c. True, 2 H (1.01 amu) + 1 O (16.01) = 18.0 amu
d. True, C6H6 -> 12(6) + 1(6) = 78 amu
17. Complete the following table
a. 0.39
b. 2.36x10^23
c. 7.08x10^23
d. 482.8
e. 1.71x10^24
f. 8.55x10^24
g. 7932.7
h. 148.3
i. 5.36x10^26
j. 46938.5
k. 425.7
l. 1276.98
m. 126.5
n. 7.77x10^23
o. 5.44x10^24
p. 0.14
q. 8.27x10^22
r. 1.65x10^24
s. 34358
t. 406.8
u. 1.23x10^2
18. Give the formula mass or the molecular mass of each substance.
- 261.67 amu
- 301.04 amu
- 286.98 amu
- 551.73 amu
19. Give the formula mass or the molecular mass of each substance.
- 273.21 amu
- 69.62 amu
- 330.04 amu
- 426.99 amu
3.6: Quantitative Information from Balanced Equations
Conceptual Problems
- What information is required to determine the mass of solute in a solution if you know the molar concentration of the solution?
Numerical Problems
- Refer to the Breathalyzer test described in Example 8. How much ethanol must be present in 89.5 mL of a person’s breath to consume all the potassium dichromate in a Breathalyzer ampul containing 3.0 mL of a 0.40 mg/mL solution of potassium dichromate?
- Phosphoric acid and magnesium hydroxide react to produce magnesium phosphate and water. If 45.00 mL of 1.50 M phosphoric acid are used in the reaction, how many grams of magnesium hydroxide are needed for the reaction to go to completion?
- Barium chloride and sodium sulfate react to produce sodium chloride and barium sulfate. If 50.00 mL of 2.55 M barium chloride is used in the reaction, how many grams of sodium sulfate are needed for the reaction to go to completion?
- How many grams of sodium phosphate are obtained in solution from the reaction of 75.00 mL of 2.80 M sodium carbonate with a stoichiometric amount of phosphoric acid? The second product is water; what is the third product? How many grams of the third product are obtained?
- How many grams of ammonium bromide are produced from the reaction of 50.00 mL of 2.08 M iron(II) bromide with a stoichiometric amount of ammonium sulfide? What is the second product? How many grams of the second product are produced?
Conceptual Answers
- The information required to determine the mass of the solution with a known molar concentration are the molar mass of the solute and the volume of the solute.
Numerical Answers
- Refer to the Breathalyzer test described in Example 8. How much ethanol must be present in 89.5 mL of a person’s breath to consume all the potassium dichromate in a Breathalyzer ampul containing 3.0 mL of a 0.40 mg/mL solution of potassium dichromate?
- 5.905 g Mg(OH)2
- 18.105 g Na2SO4
- Third Product: CO2, Grams of Third Product: 9.24 g, Grams of Sodium Phosphate: 22.26g
- Grams Produced: 20.37 grams NH4Br2, Second Product: Iron (II) Sulfide, 9.15 grams FeS
3.7: LIMITING REACTANTS
Conceptual Problems
- Engineers use conservation of mass, called a “mass balance,” to determine the amount of product that can be obtained from a chemical reaction. Mass balance assumes that the total mass of reactants is equal to the total mass of products. Is this a chemically valid practice? Explain your answer.
- Given the equation \[2H_{2\;(g)} + O_{2\;(g)} \rightarrow 2H+2O_{(g)},\] is it correct to say that 10 g of hydrogen will react with 10 g of oxygen to produce 20 g of water vapor?
- What does it mean to say that a reaction is stoichiometric?
- When sulfur is burned in air to produce sulfur dioxide, what is the limiting reactant? Explain your answer.
- Is it possible for the percent yield to be greater than the theoretical yield? Justify your answer.
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 2 () before proceeding to the Numerical Problems.
12. Write a balanced chemical equation for each reaction and then determine which reactant is in excess.
a. 2.46 g barium(s) plus 3.89 g bromine(l) in water to give barium bromide
b. 1.44 g bromine(l) plus 2.42 g potassium iodide(s) in water to give potassium bromide and iodine
c. 1.852 g of Zn metal plus 3.62 g of sulfuric acid in water to give zinc sulfate and hydrogen gas
d. 0.147 g of iron metal reacts with 0.924 g of silver acetate in water to give iron(II) acetate and silver metal
e. 3.142 g of ammonium phosphate reacts with 1.648 g of barium hydroxide in water to give ammonium hydroxide and barium phosphate
13. Under the proper conditions, ammonia and oxygen will react to form dinitrogen monoxide (nitrous oxide, also called laughing gas) and water. Write a balanced chemical equation for this reaction. Determine which reactant is in excess for each combination of reactants.
a. 24.6 g of ammonia and 21.4 g of oxygen
b. 3.8 mol of ammonia and 84.2 g of oxygen
c. 3.6 × 1024 molecules of ammonia and 318 g of oxygen
d. 2.1 mol of ammonia and 36.4 g of oxygen
14. When a piece of zinc metal is placed in aqueous hydrochloric acid, zinc chloride is produced, and hydrogen gas is evolved. Write a balanced chemical equation for this reaction. Determine which reactant is in excess for each combination of reactants.
a. 12.5 g of HCl and 7.3 g of Zn
b. 6.2 mol of HCl and 100 g of Zn
c. 2.1 × 1023 molecules of Zn and 26.0 g of HCl
d. 3.1 mol of Zn and 97.4 g of HCl
15. Determine the mass of each reactant needed to give the indicated amount of product. Be sure that the chemical equations are balanced.
a. NaI(aq) + Cl2(g) → NaCl(aq) + I2(s); 1.0 mol of NaCl
b. NaCl(aq) + H2SO4(aq) → HCl(g) + Na2SO4(aq); 0.50 mol of HCl
c. NO2(g) + H2O(l) → HNO2(aq) + HNO3(aq); 1.5 mol of HNO3
16. Determine the mass of each reactant needed to give the indicated amount of product. Be sure that the chemical equations are balanced.
a. AgNO3(aq) + CaCl2(s) → AgCl(s) + Ca(NO3)2(aq); 1.25 mol of AgCl
b. Pb(s) + PbO2(s) + H2SO4(aq) → PbSO4(s) + H2O(l); 3.8 g of PbSO4
c. H3PO4(aq) + MgCO3(s) → Mg3(PO4)2(s) + CO2(g) + H2O(l); 6.41 g of Mg3(PO4)2
17. Determine the percent yield of each reaction. Be sure that the chemical equations are balanced. Assume that any reactants for which amounts are not given are in excess. (The symbol Δ indicates that the reactants are heated.)
a. KClO3(s) \(\underrightarrow {\Delta} \) KCl(s)+O2(g);2.14 g of KClO3 produces 0.67 g of O2
b. Cu(s) + H2SO4(aq) → CuSO4(aq) + SO2(g) + H2O(l); 4.00 g of copper gives 1.2 g of sulfur dioxide
c. AgC2H3O2(aq) + Na3PO4(aq) → Ag3PO4(s) + NaC2H3O2(aq); 5.298 g of silver acetate produces 1.583 g of silver phosphate
18. Each step of a four-step reaction has a yield of 95%. What is the percent yield for the overall reaction?
19. A three-step reaction yields of 87% for the first step, 94% for the second, and 55% for the third. What is the percent yield of the overall reaction?
20. Give a general expression relating the theoretical yield (in grams) of product that can be obtained from x grams of B, assuming neither A nor B is limiting.
A + 3B → 2C
21. Under certain conditions, the reaction of hydrogen with carbon monoxide can produce methanol.
a. Write a balanced chemical equation for this reaction.
b. Calculate the percent yield if exactly 200 g of methanol is produced from exactly 300 g of carbon monoxide.
22. Chlorine dioxide is a bleaching agent used in the paper industry. It can be prepared by the following reaction:
NaClO2(s) + Cl2(g) → ClO2(aq) + NaCl(aq)
a. What mass of chlorine is needed for the complete reaction of 30.5 g of NaClO2?
b. Give a general equation for the conversion of x grams of sodium chlorite to chlorine dioxide.
23. The reaction of propane gas (CH3CH2CH3) with chlorine gas (Cl2) produces two monochloride products: CH3CH2CH2Cl and CH3CHClCH3. The first is obtained in a 43% yield and the second in a 57% yield.
a. If you use 2.78 g of propane gas, how much chlorine gas would you need for the reaction to go to completion?
b. How many grams of each product could theoretically be obtained from the reaction starting with 2.78 g of propane?
c. Use the actual percent yield to calculate how many grams of each product would actually be obtained.
24. Protactinium (Pa), a highly toxic metal, is one of the rarest and most expensive elements. The following reaction is one method for preparing protactinium metal under relatively extreme conditions:
\[ \ce{2PaI_5 (s) ->[\Delta] 2Pa (s) + 5I_2 (s)} \nonumber \]
a. Given 15.8 mg of reactant, how many milligrams of protactinium could be synthesized?
b. If 3.4 mg of Pa was obtained, what was the percent yield of this reaction?
c. If you obtained 3.4 mg of Pa and the percent yield was 78.6%, how many grams of PaI5 were used in the preparation?
25. Aniline (C6H5NH2) can be produced from chlorobenzene (C6H5Cl) via the following reaction:
C6H5Cl(l) + 2NH3(g) → C6H5NH2(l) + NH4Cl(s)
Assume that 20.0 g of chlorobenzene at 92% purity is mixed with 8.30 g of ammonia.
a. Which is the limiting reactant?
b. Which reactant is present in excess?
c. What is the theoretical yield of ammonium chloride in grams?
d. If 4.78 g of NH4Cl was recovered, what was the percent yield?
e. Derive a general expression for the theoretical yield of ammonium chloride in terms of grams of chlorobenzene reactant, if ammonia is present in excess.
26. A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following:
a. 9.36 × 1024 formula units of NaCl
b. 8.5 × 104 mol of Br2
c. 3.7 × 108 g of NaCl
Conceptual Answers
- It is not a chemically valid practice because the law of conservation of mass applies to closed/isolated systems only, so in this case of an open system converting mass into energy and allowing it to escape the system wouldn’t be valid.
- No it is not correct because in order to examine the molecular weight you need to take log of both molecules to see what there true weight is. In this case, log of O2 = 10g/(32 g/mol) = 0.3125 g/mol while log of H2 = 10g/(2 g/mol) = 5 g/mol. With that when hydrogen and oxygen react against each other ((2 x (0.3125 g/L)) x (18 g/mol H2O)) = 11.25 g of water vapor
- When a reaction is stoichiometric it means that the none of the reactants remain after being introduced leaving no excess or total leaving quantity.
- From a reaction of S + O2 -> SO2 we’re able to determine that the limiting reactant would be sulfur because the amount of O2 in the air is higher than the amount of sulfur that is burned in the reaction.
- Yes it is possible, given the equation of: percent yield = (actual yield)/(Theoretical Yield) its entirely possible to have the percent yield become greater than the theoretical yield when the product of the reaction has impurities that increase the mass compared if the product was pure.
Numerical Answers
12. Write a balanced chemical equation for each reaction and then determine which reactant is in excess.
a. Ba(s) + Br2(l) = BaBr2(aq)
Reactant in excess: Br2
b. Br2(l) + 2KI(s) = 2KBr + I2
Reactant in Excess: Br2
c. Zn + H2SO4 = ZnSO4 + H2
Reactant in excess: H2SO4
d. Fe + 2 AgC2H3O2 = Fe(C2H3O2)2 + 2 Ag
Reactant in excess: AgC2H3O2
e. 2 (NH4)3PO4 + 3 Ba(OH)2 = 6 NH4OH + Ba3(PO4)2
Reactant in excess: Ba(OH)2
13. The balanced chemical equation for this reaction is
2NH3 + 2O2 → N2O + 3H2O
a. NH3
b. NH3
c. O2
d. NH3
14. a. Excess: HCl
b. Excess: HCl
c. Excess: HCl
d. Excess: Zn
15.
a. 150 g NaI and 35 g Cl2
b. 29 g NaCl and 25 g H2SO4
c. 140 g NO2 and 27 g H2O
16.
a. 2 AgNO3(aq) + CaCl2(s) = 2 AgCl(s) + Ca(NO3)2(aq); Mass of Reactant: 212.34g AgNO3 and 69.4 g CaCl2
b. Pb(s) + PbO2(s) + 2 H2SO4(aq) = 2 PbSO4(s) + 2 H2O(l); Mass of Reactant: 1.3g Pb, 1.5g PbO2, 1.2g H2SO4
c. 2 H3PO4(aq) + 3 MgCO3(s) = Mg3(PO4)2(s) + 3 CO2(g) + 3 H2O(l); Mass of Reactant: 6.17 g of MgCO3, 4.78g of H3PO4
17.
a. 80%
b. 30%
c. 35.7%
18. 81%
19. 45%.
20. (x/molecular mass of B) x (2/3) x (molecular mass of C)
21.
a. CO + 2H2 → CH3OH
b. 58.28%
22. 2 NaClO2(s) + Cl2(g) → ClO2(aq) + NaCl(aq)
a. 11.9 g Cl2
b. (0.7458g= Grams of ClO2 = (x/(90.44 g/mol)) x (2/2) x (67.45 g ClO2/ 1 mol ClO2
23.
a. 2.24 g Cl2
b. 4.95 g
c. 2.13 g CH3CH2CH2Cl plus 2.82 g CH3CHClCH3
24. a. 4.14mg Pa
b. 82.1%
c. 0.0162 g PaI5
25.
a. chlorobenzene
b. ammonia
c. 8.74 g ammonium chloride.
d. 55%
e. \(Theoretical \, yield \, (NH_4Cl) = {mass \, of \, chlorobenzene \, (g) \times 0.92 \times \times 53.49 \, g/mol \over 112.55 \, g/mol} \)
26.
a. 6619.19g Cl2
b. 6.77 x 106 g Cl2
c. 2.52 x 108 g Cl2
Conceptual Problems II
- Engineers use conservation of mass, called a “mass balance,” to determine the amount of product that can be obtained from a chemical reaction. Mass balance assumes that the total mass of reactants is equal to the total mass of products. Is this a chemically valid practice? Explain your answer.
- Given the equation \[2H_{2\;(g)} + O_{2\;(g)} \rightarrow 2H+2O_{(g)},\] is it correct to say that 10 g of hydrogen will react with 10 g of oxygen to produce 20 g of water vapor?
- What does it mean to say that a reaction is stoichiometric?
- When sulfur is burned in air to produce sulfur dioxide, what is the limiting reactant? Explain your answer.
- Is it possible for the percent yield to be greater than the theoretical yield? Justify your answer.
Numerical Problems II
Please be sure you are familiar with the topics discussed in Essential Skills 2 () before proceeding to the Numerical Problems.
17. Determine the percent yield of each reaction. Be sure that the chemical equations are balanced. Assume that any reactants for which amounts are not given are in excess. (The symbol Δ indicates that the reactants are heated.)
a. KClO3(s) \(\underrightarrow {\Delta} \) KCl(s)+O2(g);2.14 g of KClO3 produces 0.67 g of O2
b. Cu(s) + H2SO4(aq) → CuSO4(aq) + SO2(g) + H2O(l); 4.00 g of copper gives 1.2 g of sulfur dioxide
c. AgC2H3O2(aq) + Na3PO4(aq) → Ag3PO4(s) + NaC2H3O2(aq); 5.298 g of silver acetate produces 1.583 g of silver phosphate
18. Each step of a four-step reaction has a yield of 95%. What is the percent yield for the overall reaction?
19. A three-step reaction yields of 87% for the first step, 94% for the second, and 55% for the third. What is the percent yield of the overall reaction?
20. Give a general expression relating the theoretical yield (in grams) of product that can be obtained from x grams of B, assuming neither A nor B is limiting.
A + 3B → 2C
21. Under certain conditions, the reaction of hydrogen with carbon monoxide can produce methanol.
a. Write a balanced chemical equation for this reaction.
b. Calculate the percent yield if exactly 200 g of methanol is produced from exactly 300 g of carbon monoxide.
22. Chlorine dioxide is a bleaching agent used in the paper industry. It can be prepared by the following reaction:
NaClO2(s) + Cl2(g) → ClO2(aq) + NaCl(aq)
a. What mass of chlorine is needed for the complete reaction of 30.5 g of NaClO2?
b. Give a general equation for the conversion of x grams of sodium chlorite to chlorine dioxide.
23. The reaction of propane gas (CH3CH2CH3) with chlorine gas (Cl2) produces two monochloride products: CH3CH2CH2Cl and CH3CHClCH3. The first is obtained in a 43% yield and the second in a 57% yield.
a. If you use 2.78 g of propane gas, how much chlorine gas would you need for the reaction to go to completion?
b. How many grams of each product could theoretically be obtained from the reaction starting with 2.78 g of propane?
c. Use the actual percent yield to calculate how many grams of each product would actually be obtained.
24. Protactinium (Pa), a highly toxic metal, is one of the rarest and most expensive elements. The following reaction is one method for preparing protactinium metal under relatively extreme conditions:
\[ 2PaI_5 (s) \underrightarrow {\Delta} 2Pa (s) + 5I_2 (s) \]
a. Given 15.8 mg of reactant, how many milligrams of protactinium could be synthesized?
b. If 3.4 mg of Pa was obtained, what was the percent yield of this reaction?
c. If you obtained 3.4 mg of Pa and the percent yield was 78.6%, how many grams of PaI5 were used in the preparation?
25. Aniline (C6H5NH2) can be produced from chlorobenzene (C6H5Cl) via the following reaction:
C6H5Cl(l) + 2NH3(g) → C6H5NH2(l) + NH4Cl(s)
Assume that 20.0 g of chlorobenzene at 92% purity is mixed with 8.30 g of ammonia.
a. Which is the limiting reactant?
b. Which reactant is present in excess?
c. What is the theoretical yield of ammonium chloride in grams?
d. If 4.78 g of NH4Cl was recovered, what was the percent yield?
e. Derive a general expression for the theoretical yield of ammonium chloride in terms of grams of chlorobenzene reactant, if ammonia is present in excess.
26. A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine. Write the chemical equation for this reaction. Then assume an 89% yield and calculate the mass of chlorine given the following:
a. 9.36 × 1024 formula units of NaCl
b. 8.5 × 104 mol of Br2
c. 3.7 × 108 g of NaCl
Numerical Answers II
17.
a. 80%
b. 30%
c. 35.7%
19. 45%.
21.
a. CO + 2H2 → CH3OH
b. 58.28%
23.
a. 2.24 g Cl2
b. 4.95 g
c. 2.13 g CH3CH2CH2Cl plus 2.82 g CH3CHClCH3
25.
a. chlorobenzene
b. ammonia
c. 8.74 g ammonium chloride.
d. 55%
e. \(Theoretical \, yield \, (NH_4Cl) = {mass \, of \, chlorobenzene \, (g) \times 0.92 \times \times 53.49 \, g/mol \over 112.55 \, g/mol} \)