Objectives
After completing this section, you should be able to:
- Name a substituted or unsubstituted cycloalkane, given its Kekulé structure, shorthand structure or condensed structure.
- Draw the Kekulé, shorthand or condensed structure for a substituted or unsubstituted cycloalkane, given its IUPAC name.
Key Terms
Make certain that you can define, and use in context, the key terms below.
Study Notes
Provided that you have mastered the IUPAC system for naming alkanes, you should find that the nomenclature of cycloalkanes does not present any particular difficulties.
Many organic compounds found in nature contain rings of carbon atoms. These compounds are known as cycloalkanes. Cycloalkanes only contain carbon-hydrogen bonds and carbon-carbon single bonds. The simplest examples of this class consist of a single, un-substituted carbon ring, and these form a homologous series similar to the unbranched alkanes.
Like alkanes, cycloalkane molecules are often drawn as skeletal structures in which each intersection between two lines is assumed to have a carbon atom with its corresponding number of hydrogens. Cyclohexane, one of the most common cycloalkanes is shown below as an example.

Cyclic hydrocarbons have the prefix "cyclo-". The IUPAC names, molecular formulas, and skeleton structures of the cycloalkanes with 3 to 10 carbons are given in Table \(\PageIndex{1}\). Note that the general formula for a cycloalkane composed of \(\ce{n}\) carbons is \(\ce{C_{n}H_{2n}}\), and not \(\ce{C_{n}H_{2n+2}}\) as for alkanes. Although a cycloalkane has two fewer hydrogens than the equivalent alkane, each carbon is bonded to four other atoms so are still considered to be saturated with hydrogen.
Table \(\PageIndex{1}\): Examples of Simple Cycloalkanes
| Cycloalkane |
Molecular Formula |
Skeleton Structure |
| Cyclopropane |
C3H6 |
 |
| Cyclobutane |
C4H8 |
 |
| Cyclopentane |
C5H10 |
 |
| Cyclohexane |
C6H12 |
 |
| Cycloheptane |
C7H14 |
 |
| Cyclooctane |
C8H16 |
 |
| Cyclononane |
C9H18 |
 |
| Cyclodecane |
C10H20 |
 |
IUPAC Rules for Nomenclature
The naming of substituted cycloalkanes follows the same basic steps used in naming alkanes.
- Determine the parent chain.
- Number the substituents of the ring beginning at one substituent so that the nearest substituent is numbered the lowest possible. If there are multiple choices that are still the same, go to the next substituent and give it the lowest number possible.
- Name the substituents and place them in alphabetical order.
More specific rules for naming substituted cycloalkanes with examples are given below.
- Determine the cycloalkane to use as the parent. If there is an alkyl straight chain that has a greater number of carbons than the cycloalkane, then the alkyl chain must be used as the primary parent chain. Cycloalkanes substituents have an ending "-yl". If there are two cycloalkanes in the molecule, use the cycloalkane with the higher number of carbons as the parent.
Example \(\PageIndex{1}\)

The longest straight chain contains 10 carbons, compared with cyclopropane, which only contains 3 carbons. The parent chain in this molecule is decane and cyclopropane is a substituent. The name of this molecule is 3-cyclopropyl-6-methyldecane.
Example \(\PageIndex{2}\)
Name the cycloalkane structure.

Solution
There are two different cycloalkanes in this molecule. Because it contains more carbons, the cyclopentane ring will be named as the parent chain. The smaller ring, cyclobutane, is named as a substituent on the parent chain. The name of this molecule is cyclobutylcyclopentane.
- When there is only one substituent on the ring, the ring carbon attached to the substituent is automatically carbon #1. Indicating the number of the carbon with the substituent in the name is optional.
Example \(\PageIndex{3}\)
 |
 |
| 1-chlorocyclobutane or cholorocyclobutane |
1-propylcyclohexane or propylcyclohexane |
- If there are multiple substituents on the ring, number the carbons of the cycloalkane so that the carbons with substituents have the lowest possible number. A carbon with multiple substituents should have a lower number than a carbon with only one substituent or functional group. One way to make sure that the lowest number possible is assigned is to number the carbons so that when the numbers corresponding to the substituents are added, their sum is the lowest possible.
- When naming the cycloalkane, the substituents must be placed in alphabetical order. Remember the prefixes di-, tri-, etc. , are not used for alphabetization.
Example \(\PageIndex{4}\)

In this example, the ethyl or the methyl subsistent could be attached to carbon one. The ethyl group attachment is assigned carbon 1 because ethyl comes before methyl alphabetically. After assigning carbon 1 the cyclohexane ring can be numbered going clockwise or counterclockwise. When looking at the numbers produced going clockwise produces lower first substituent numbers (1,3) than when numbered counterclockwise (1,5). So the correct name is 1-ethyl-3-methylcyclohexane.
Example \(\PageIndex{5}\)
Name the following structure using IUPAC rules.

Solution
Remember when dealing with cycloalkanes with more than two substituents, finding the lowest possible 2nd substituent numbering takes precedence. Consider a numbering system with each substituent attachment point as being carbon one. Compare them and whichever produces the lowest first point of difference will be correct.
The first structure would have 1,4 for the relationship between the first two groups. The next structure would have 1,3. The final 2 structures both have 1,2 so those are preferable to the first two. Now we have to determine which is better between the final 2 structures. The 3rd substituent on structure 3 would be at the 5 position leading to 1,2,5 while in the final structure the 3rd methyl group is on carbon 4 leading to 1,2,4. This follows the rules of giving the lowest numbers at the first point of difference.

The correct name for the molecule is 4-Bromo-1,2-dimethylcyclohexane.
Example \(\PageIndex{6}\)

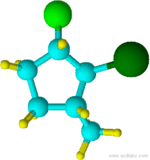
2-bromo-1-chloro-3-methylcyclopentane
Notice that "b" of bromo alphabetically precedes the "m" of methyl. Also, notice that the chlorine attachment point is assigned carbon 1 because it comes first alphabetically and the overall sum of numbers would be the same if the methyl attachment carbon was assigned as 1 and the chlorine attachment as 3.
Example \(\PageIndex{7}\)

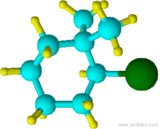
(2-bromo-1,1-dimethylcyclohexane)
Although "di" alphabetically precedes "f", "di" is not used in determining the alphabetical order.
Example \(\PageIndex{8}\)

2-fluoro-1,1,-dimethylcyclohexane NOT 1,1-dimethyl-2-fluorocyclohexane also 2-fluoro-1,1,-dimethylcyclohexane NOT 1-fluoro-2,2-dimethylcyclohexane (as that would give a larger number to the first point of difference)
Although "di" alphabetically precedes "f", "di" is not used in determining the alphabetical order of the substituents. Notice that the attachment point of the two methyl groups is assigned carbon 1 despite the fact that fluorine comes first alphabetically. This is because this assignment allows for a lower overall numbering of substituents, so assigning alphabetical priority is not necessary.
Polycyclic Compounds
Hydrocarbons having more than one ring are common, and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of \(n\) carbon atoms associated with \(m\) rings the formula is: \(\ce{C_{n}H_{2n + 2 - 2m}}\). The structural relationship of rings in a polycyclic compound can vary. They may be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following table.
Table \(\PageIndex{2}\): Examples of Isomeric \(\ce{C_8H_{14}}\) Bicycloalkanes
| Isolated Rings |
Spiro Rings |
Fused Rings |
Bridged Rings |
| No common atoms |
One common atom |
One common bond |
Two common atoms |
 |
 |
 |
 |
Polycyclic compounds, like cholesterol shown below, are biologically important and typically have common names accepted by IUPAC. However, the common names do not generally follow the basic IUPAC nomenclature rules, and will not be covered here.

Cholesterol (polycyclic)
Exercise \(\PageIndex{1}\)
Give the IUPAC names for the following cycloalkane structures.

- Answer
-
- 1,3-Dimethylcyclohexane
- 2-Cyclopropylbutane
- 1-Ethyl-3-methylcyclooctane
- 1-Bromo-3-methylcyclobutane
- 1,2,4-Triethylcycloheptane
- 1-Chloro-2,4-dimethylcyclopentane
Exercise \(\PageIndex{2}\)
Draw the structures for the IUPAC names below.
- 2,3-dicyclopropylpentane
- 1,2,3-triethylcyclopentane
- 3-cyclobutyl-2-methylhexane
- 2-bromo-1-chloro-4-methylcyclohexane
- 1-bromo-5-propylcyclododecane
- Answer
-

Objectives
After completing this section, you should be able to
- draw structural formulas that distinguish between cis and trans disubstituted cycloalkanes.
- construct models of cis and trans disubstituted cycloalkanes using ball-and-stick molecular models.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- constitutional isomer
- stereoisomer
- cis-trans isomers
Previously, constitutional isomers have been defined as molecules that have the same molecular formula, but different atom connectivity. In this section, a new class of isomers, stereoisomers, will be introduced. Stereoisomers are molecules that have the same molecular formula, the same atom connectivity, but they differ in the relative spatial orientation of the atoms.
Cycloalkanes are similar to open-chain alkanes in many respects. They both tend to be nonpolar and relatively inert. One important difference, is that cycloalkanes have much less freedom of movement than open-chain alkanes. As discussed in Sections 3.6 and 3.7, open-chain alkanes are capable of rotation around their carbon-carbon sigma bonds. The ringed structures of cycloalkanes prevent such free rotation, causing them to be more rigid and somewhat planar.
Di-substituted cycloalkanes are one class of molecules that exhibit stereoisomerism. 1,2-dibromocyclopentane can exist as two different stereoisomers: cis-1,2-dibromocyclopentane and trans-1,2-dibromocyclopentane. The cis-1,2-dibromocyclopentane and trans-1,2-dibromocyclopentane stereoisomers of 1,2-dibromocyclopentane are shown below. Both molecules have the same molecular formula and the same atom connectivity. They differ only in the relative spatial orientation of the two bromines on the ring. In cis-1,2-dibromocyclopentane, both bromine atoms are on the same "face" of the cyclopentane ring, while in trans-1,2-dibromocyclopentane, the two bromines are on opposite faces of the ring. Stereoisomers require an additional nomenclature prefix be added to the IUPAC name in order to indicate their spatial orientation. Di-substituted cycloalkane stereoisomers are designated by the nomenclature prefixes cis (Latin, meaning on this side) and trans (Latin, meaning across).


Representing 3D Structures
By convention, chemists use heavy, wedge-shaped bonds to indicate a substituent located above the plane of the ring (coming out of the page), a dashed line for bonds to atoms or groups located below the ring (going back into the page), and solid lines for bonds in the plane of the page.




In general, if any two sp3 carbons in a ring have two different substituent groups (not counting other ring atoms) cis/trans stereoisomerism is possible. However, the cis/trans designations are not used if both groups are on the same carbon. For example, the chlorine and the methyl group are on the same carbon in 1-chloro-1-methylcyclohexane and the trans prefix should not be used.

If more than two ring carbons have substituents, the stereochemical notation distinguishing the various isomers becomes more complex and the prefixes cis and trans cannot be used to formally name the molecule. However, the relationship of any two substituents can be informally described using cis or trans. For example, in the tri-substituted cyclohexane below, the methyl group is cis to the ethyl group, and also trans to the chlorine. However, the entire molecule cannot be designated as either a cis or trans isomer. Later sections will describe how to name these more complex molecules (5.5: Sequence Rules for Specifying Configuration)

Example \(\PageIndex{1}\)
Name the following cycloalkanes:
_cis-1%252C4-dibromocyclohexane_and_b)_trans-1%252C2-dimethylcyclopropane.svg?revision=1&size=bestfit&width=341&height=90)
Solution
These two example represent the two main ways of showing spatial orientation in cycloalkanes.
- In example "a" the cycloalkane is shown as being flat and in the plane of the page. The positioning of the substituents is shown by using dash-wedge bonds. Cis/trans positioning can be determined by looking at the type of bonds attached to the substituents. If the substituents are both on the same side of the ring (Cis) they would both have either dash bonds or wedge bonds. If the the substituents are on opposite side of the ring (Trans) one substituent would have a dash bond and the other a wedge bond. Because both bromo substituents have a wedge bond they are one the same side of the ring and are cis. The name of this molecule is cis-1,4-Dibromocyclohexane.
- Example "b" shows the cycloalkane ring roughly perpendicular to the plane of the page. When this is done, the upper and lower face of the ring is defined and each carbon in the ring will have a bond one the upper face and a bond on the lower face. Cis substituents will either both be on the upper face or the lower face. Trans substituents will have one on the upper face and one one the lower face. In example "b", one of the methyl substituents is on the upper face of the ring and one is on the lower face which makes them trans to each other. The name of this molecule is trans-1,2-Dimethylcyclopropane.
Exercise \(\PageIndex{2}\)
Draw the following molecules:
- trans-1,3-dimethylcyclohexane
- trans-1,2-dibromocyclopentane
- cis-1,3-dichlorocyclobutane
- Answer
-

Exercise \(\PageIndex{3}\)
Cis/Trans nomenclature can be used to describe the relative positioning of substituents on molecules with more complex ring structures. The molecule below is tesosterone, the primary male sex hormone. Is the OH and the adjacent methyl group cis or trans to each other? What can you deduce about the relative positions of the indicated hydrogens?

- Answer
-
Both the OH and the methyl group have wedge bonds. This implies that they are both on the same side of the testosterone ring making them cis. Two of the hydrogens have wedge bonds while one has a wedge. This means two of the hydrogens are on one side of the testosterone ring while one is on the other side.
Exercise \(\PageIndex{4}\)
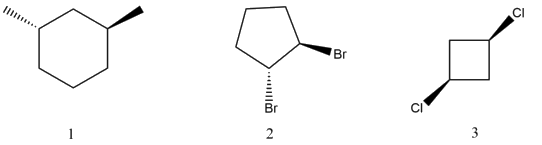
Name the following compounds:



- Answer
-
Cis-1-Bromo-3-Chlorocyclobutane
Trans-1,4-Dimethylcyclooctane
Trans-1-Bromo-3-ethylcyclopentane
Exercise \(\PageIndex{5}\)
Draw the following molecules:
- trans-1,3-dimethylcyclohexane
- trans-1,2-dibromocyclopentane
- cis-1,3-dichlorocyclobutane
- Answer
-