Chapter 4.3: Chemical Formulas
- Page ID
- 18846
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Learning Objectives
- To describe the composition of a chemical compound.
Chapter 1 introduced some of the fundamental concepts of chemistry, with particular attention to the basic properties of atoms and elements. These entities are the building blocks of all substances we encounter, yet most common substances do not consist of only pure elements or individual atoms. Instead, nearly all substances are chemical compounds or mixtures of chemical compounds. Although there are only about 115 elements (of which about 86 occur naturally), millions of chemical compounds are known, with a tremendous range of physical and chemical properties. Consequently, the emphasis of modern chemistry (and this text) is on understanding the relationship between the structures and properties of chemical compounds.
Antoine Lavoisier expressed the need for a systematic chemical nomenclature in the Preface to his Elements of Chemistry
The impossibility of separating the nomenclature of a science from the science itself, is owing to this, that every branch of physical science must consist of three things; the series of facts which are the objects of the science, the ideas which represent these facts, and the words by which these ideas are expressed. Like three impressions of the same seal, the word ought to produce the idea, and the idea to be a picture of the fact. And, as ideas are preserved and communicated by means of words, it necessarily follows that we cannot improve the language of any science without at the same time improving the science itself; neither can we, on the other hand, improve a science, without improving the language or nomenclature which belongs to it. However certain the facts of any science may be, and, however just the ideas we may have formed of these facts, we can only communicate false impressions to others, while we want words by which these may be properly expressed
In this chapter we introduce you to chemical nomenclature—the language of chemistry—that will enable you to recognize and name the most common kinds of compounds. An understanding of chemical nomenclature not only is essential for your study of chemistry but also has other benefits—for example, it helps you understand the labels on products found in the supermarket and the pharmacy.
When chemists synthesize a new compound, they may not yet know its molecular or structural formula. In such cases, they usually begin by determining its empirical formulaA formula for a compound that consists of the atomic symbol for each component element accompanied by a subscript indicating the relative number of atoms of that element in the compound, reduced to the smallest whole numbers., the relative numbers of atoms of the elements in a compound, reduced to the smallest whole numbers. Because the empirical formula is based on experimental measurements of the numbers of atoms in a sample of the compound, it shows only the ratios of the numbers of the elements present.
Because ionic compounds do not contain discrete molecules, empirical formulas are used to indicate their compositions. All compounds, whether ionic or covalent, must be electrically neutral. Consequently, the positive and negative charges in a formula unit must exactly cancel each other. If the cation and the anion have charges of equal magnitude, such as Na+ and Cl−, then the compound must have a 1:1 ratio of cations to anions, and the empirical formula must be NaCl. If the charges are not the same magnitude, then a cation:anion ratio other than 1:1 is needed to produce a neutral compound. In the case of Mg2+ and Cl−, for example, two Cl− ions are needed to balance the two positive charges on each Mg2+ ion, giving an empirical formula of MgCl2. Similarly, the formula for the ionic compound that contains Na+ and O2− ions is Na2O.
A characteristic of chemical nomenclature is that it is both descriptive and parsimonious. For ionic compounds our knowledge of the electronic structure of each ion tells us what the charge on the ion is and since we know that the formula unit must have no charge we do not have to provide information about the number of the anions or cations in the formula unit.
Note the Pattern
Ionic compounds do not contain discrete molecules, so empirical formulas are used to indicate their compositions.
Binary Ionic Compounds
An ionic compound that contains only two elements, one present as a cation and one as an anion, is called a binary ionic compoundAn ionic compound that contains only two elements, one present as a cation and one as an anion.. One example is MgCl2, a coagulant used in the preparation of tofu from soybeans. For binary ionic compounds, the subscripts in the empirical formula can also be obtained by crossing charges: use the absolute value of the charge on one ion as the subscript for the other ion. This method is shown schematically as follows:
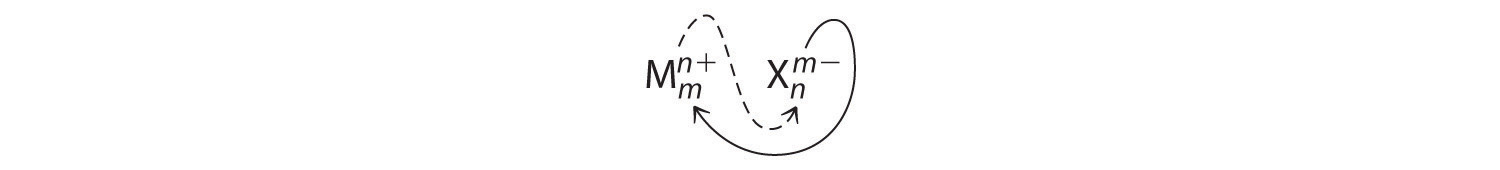
Crossing charges. One method for obtaining subscripts in the empirical formula is by crossing charges.
When crossing charges, you will sometimes find it necessary to reduce the subscripts to their simplest ratio to write the empirical formula. Consider, for example, the compound formed by Mg2+ and O2−. Using the absolute values of the charges on the ions as subscripts gives the formula Mg2O2:
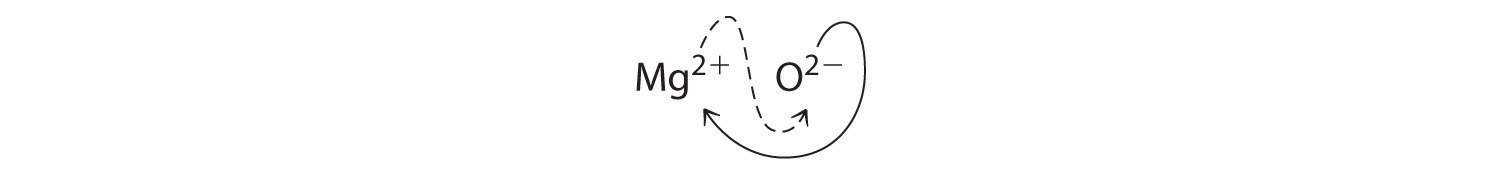
This simplifies to its correct empirical formula MgO. The empirical formula has one Mg2+ ion and one O2− ion.
Example 3
Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair.
- Ga3+ and As3−
- Eu3+ and O2−
- calcium and chlorine
Given: ions or elements
Asked for: empirical formula for binary ionic compound
Strategy:
A If not given, determine the ionic charges based on the location of the elements in the periodic table.
B Use the absolute value of the charge on each ion as the subscript for the other ion. Reduce the subscripts to the lowest numbers to write the empirical formula. Check to make sure the empirical formula is electrically neutral.
Solution:
-
B Using the absolute values of the charges on the ions as the subscripts gives Ga3As3:
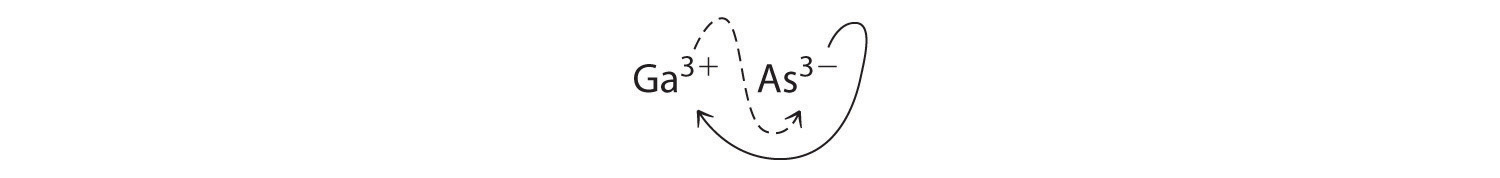
Reducing the subscripts to the smallest whole numbers gives the empirical formula GaAs, which is electrically neutral [+3 + (−3) = 0]. Alternatively, we could recognize that Ga3+ and As3− have charges of equal magnitude but opposite signs. One Ga3+ ion balances the charge on one As3− ion, and a 1:1 compound will have no net charge. Because we write subscripts only if the number is greater than 1, the empirical formula is GaAs. GaAs is gallium arsenide, which is widely used in the electronics industry in transistors and other devices.
-
B Because Eu3+ has a charge of +3 and O2− has a charge of −2, a 1:1 compound would have a net charge of +1. We must therefore find multiples of the charges that cancel. We cross charges, using the absolute value of the charge on one ion as the subscript for the other ion:
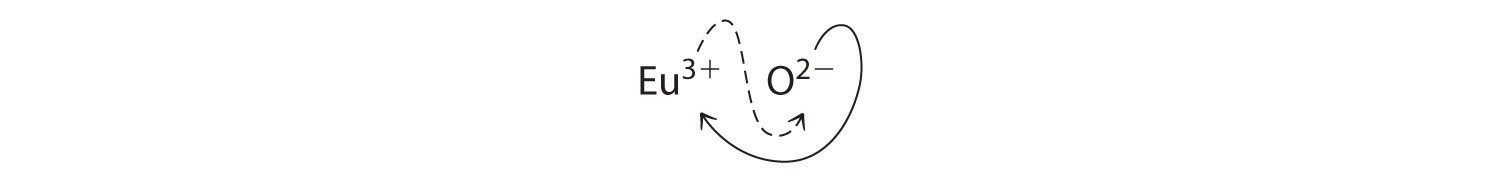
The subscript for Eu3+ is 2 (from O2−), and the subscript for O2− is 3 (from Eu3+), giving Eu2O3; the subscripts cannot be reduced further. The empirical formula contains a positive charge of 2(+3) = +6 and a negative charge of 3(−2) = −6, for a net charge of 0. The compound Eu2O3 is neutral. Europium oxide is responsible for the red color in television and computer screens.
-
A Because the charges on the ions are not given, we must first determine the charges expected for the most common ions derived from calcium and chlorine. Calcium lies in group 2, so it should lose two electrons to form Ca2+. Chlorine lies in group 17, so it should gain one electron to form Cl−.
B Two Cl− ions are needed to balance the charge on one Ca2+ ion, which leads to the empirical formula CaCl2. We could also cross charges, using the absolute value of the charge on Ca2+ as the subscript for Cl and the absolute value of the charge on Cl− as the subscript for Ca:
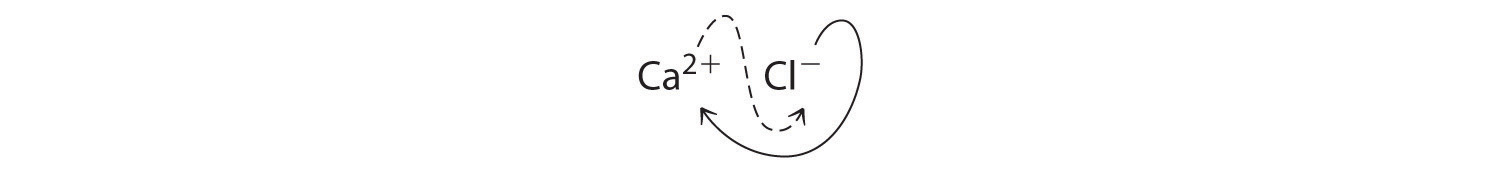
The subscripts in CaCl2 cannot be reduced further. The empirical formula is electrically neutral [+2 + 2(−1) = 0]. This compound is calcium chloride, one of the substances used as “salt” to melt ice on roads and sidewalks in winter.
Exercise
Write the empirical formula for the simplest binary ionic compound formed from each ion or element pair.
- Li+ and N3−
- Al3+ and O2−
- lithium and oxygen
Answer:
- Li3N
- Al2O3
- Li2O
Consistent with a tendency to have the same number of electrons as the nearest noble gas, when forming ions, elements in groups 1, 2, and 3 tend to lose one, two, and three electrons, respectively, to form cations, such as Na+ and Mg2+. They then have the same number of electrons as the nearest noble gas: neon. Similarly, K+, Ca2+, and Sc3+ have 18 electrons each, like the nearest noble gas: argon. In addition, the elements in group 13 lose three electrons to form cations, such as Al3+, again attaining the same number of electrons as the noble gas closest to them in the periodic table. Because the lanthanides and actinides formally belong to group 3, the most common ion formed by these elements is M3+, where M represents the metal. Conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such as Cl−, S2−, and P3−. Ions such as these, which contain only a single atom, are called monatomicions. The names of the single atom cations are simply the name of the metal from which they are derived. The names of the single atom anions add the suffix -ide to the first syllable of the atom, for example oxide, chloride, nitride, etc.
You can predict the charges of most monatomic ions derived from the main group elements by simply looking at the periodic table and counting how many columns an element lies from the extreme left or right. For example, you can predict that barium (in group 2) will form Ba2+ to have the same number of electrons as its nearest noble gas, xenon, that oxygen (in group 16) will form O2− to have the same number of electrons as neon, and cesium (in group 1) will form Cs+ to also have the same number of electrons as xenon. Note that this method does not usually work for most of the transition metals. Some common monatomic ions are in Table 4.3.1
Note the Pattern
Elements in groups 1, 2, and 3 tend to form 1+, 2+, and 3+ ions, respectively; elements in groups 15, 16, and 17 tend to form 3−, 2−, and 1− ions, respectively.
Table 4.3.1 Some Common Monatomic Ions and Their Names
| Group 1 | Group 2 | Group 3 | Group 13 | Group 15 | Group 16 | Group 17 |
|---|---|---|---|---|---|---|
|
Li+ lithium |
Be2+ beryllium |
N3− nitride (azide) |
O2− oxide |
F− fluoride |
||
|
Na+ sodium |
Mg2+ magnesium |
Al3+ aluminum |
P3− phosphide |
S2− sulfide |
Cl− chloride |
|
|
K+ potassium |
Ca2+ calcium |
Sc3+ scandium |
Ga3+ gallium |
As3− arsenide |
Se2− selenide |
Br− bromide |
|
Rb+ rubidium |
Sr2+ strontium |
Y3+ yttrium |
In3+ indium |
Te2− telluride |
I− iodide |
|
|
Cs+ cesium |
Ba2+ barium |
La3+ lanthanum |
Example 4
Given: element
A Identify the group in the periodic table to which the element belongs. Based on its location in the periodic table, decide whether the element is a metal, which tends to lose electrons; a nonmetal, which tends to gain electrons; or a semimetal, which can do either.
- A Aluminum is a metal in group 13; consequently, it will tend to lose electrons. B The nearest noble gas to aluminum is neon. Aluminum will lose three electrons to form the Al3+ ion, which has the same number of electrons as neon.
- A Selenium is a nonmetal in group 16, so it will tend to gain electrons. B The nearest noble gas is krypton, so we predict that selenium will gain two electrons to form the Se2− ion, which has the same number of electrons as krypton.
- A Yttrium is in group 3, and elements in this group are metals that tend to lose electrons. B The nearest noble gas to yttrium is krypton, so yttrium is predicted to lose three electrons to form Y3+, which has the same number of electrons as krypton.
- calcium, used to prevent osteoporosis
- iodine, required for the synthesis of thyroid hormones
- zirconium, widely used in nuclear reactors
- <p class="para" id="av_1.0-ch06_s01_s03_p17" trebuchet="" ms',="" sans-serif;="" visibility:="" visible;"="">Answer:<ol class="orderedlist" id="av_1.0-ch06_s01_s03_l04" trebuchet="" ms',="" sans-serif;"="">
- Ca2+
- I−
- Zr4+
Polyatomic Ions
Polyatomic ionsA group of two or more atoms that has a net electrical charge. are groups of atoms that bear a net electrical charge, although the atoms in a polyatomic ion are held together by covalent bonds, a sharing of electrons. We will discuss covalent bonding in the next chapter. Just as there are many more kinds of molecules than simple elements, there are many more kinds of polyatomic ions than monatomic ions. Two examples of polyatomic cations are the ammonium (NH4+) and the methylammonium (CH3NH3+) ions. These are formed by adding a proton (the hydrogen nucleus) to the lone pair in ammonia and methylammonia. Polyatomic anions are much more numerous than polyatomic cations; some common examples are in Table 4.3.2 .
Table 4.3.2 Common Polyatomic Ions and Their Names
| Formula | Name of Ion |
|---|---|
| NH4+ | ammonium |
| CH3NH3+ | methylammonium |
| OH− | hydroxide |
| O22− | peroxide |
| CN− | cyanide |
| SCN− | thiocyanate |
| NO2− | nitrite |
| NO3− | nitrate |
| CO32− | carbonate |
| HCO3− | hydrogen carbonate, or bicarbonate |
| SO32− | sulfite |
| SO42− | sulfate |
| HSO4− | hydrogen sulfate, or bisulfate |
| PO43− | phosphate |
| HPO42− | hydrogen phosphate |
| H2PO4− | dihydrogen phosphate |
| ClO− | hypochlorite |
| ClO2− | chlorite |
| ClO3− | chlorate |
| ClO4− | perchlorate |
| MnO4− | permanganate |
| CrO42− | chromate |
| Cr2O72− | dichromate |
| C2O42− | oxalate |
| HCO2− | formate |
| CH3CO2− | acetate |
| C6H5CO2− | benzoate |
The method we used to predict the empirical formulas for ionic compounds that contain monatomic ions can also be used for compounds that contain polyatomic ions. The overall charge on the cations must balance the overall charge on the anions in the formula unit. Thus K+ and NO3− ions combine in a 1:1 ratio to form KNO3 (potassium nitrate or saltpeter), a major ingredient in black gunpowder. Similarly, Ca2+ and SO42− form CaSO4 (calcium sulfate), which combines with varying amounts of water to form gypsum and plaster of Paris. The polyatomic ions NH4+ and NO3− form NH4NO3 (ammonium nitrate), which is a widely used fertilizer and, in the wrong hands, an explosive. One example of a compound in which the ions have charges of different magnitudes is calcium phosphate, which is composed of Ca2+ and PO43− ions; it is a major component of bones. The compound is electrically neutral because the ions combine in a ratio of three Ca2+ ions [3(+2) = +6] for every two ions [2(−3) = −6], giving an empirical formula of Ca3(PO4)2; the parentheses around PO4 in the empirical formula indicate that it is a polyatomic ion. Writing the formula for calcium phosphate as Ca3P2O8 gives the correct number of each atom in the formula unit, but it obscures the fact that the compound contains readily identifiable PO43− ions.
Example 5
Write the empirical formula for the compound formed from each ion pair.
- Na+ and HPO42−
- potassium cation and cyanide anion
- calcium cation and hypochlorite anion
Given: ions
Asked for: empirical formula for ionic compound
Strategy:
A If it is not given, determine the charge on a monatomic ion from its location in the periodic table. Use Table 4.3.2 to find the charge on a polyatomic ion.
B Use the absolute value of the charge on each ion as the subscript for the other ion. Reduce the subscripts to the smallest whole numbers when writing the empirical formula.
Solution:
- B Because HPO42− has a charge of −2 and Na+ has a charge of +1, the empirical formula requires two Na+ ions to balance the charge of the polyatomic ion, giving Na2HPO4. The subscripts are reduced to the lowest numbers, so the empirical formula is Na2HPO4. This compound is sodium hydrogen phosphate, which is used to provide texture in processed cheese, puddings, and instant breakfasts.
- A The potassium cation is K+, and the cyanide anion is CN−. B Because the magnitude of the charge on each ion is the same, the empirical formula is KCN. Potassium cyanide is highly toxic, and at one time it was used as rat poison. This use has been discontinued, however, because too many people were being poisoned accidentally.
- A The calcium cation is Ca2+, and the hypochlorite anion is ClO−. B Two ClO− ions are needed to balance the charge on one Ca2+ ion, giving Ca(ClO)2. The subscripts cannot be reduced further, so the empirical formula is Ca(ClO)2. This is calcium hypochlorite, the “chlorine” used to purify water in swimming pools.
Exercise
Write the empirical formula for the compound formed from each ion pair.
- Ca2+ and H2PO4−
- sodium cation and bicarbonate anion
- ammonium cation and sulfate anion
Answer:
- Ca(H2PO4)2: calcium dihydrogen phosphate is one of the ingredients in baking powder.
- NaHCO3: sodium bicarbonate is found in antacids and baking powder; in pure form, it is sold as baking soda.
- (NH4)2SO4: ammonium sulfate is a common source of nitrogen in fertilizers.
Hydrates
Many ionic compounds occur as hydratesA compound that contains specific ratios of loosely bound water molecules, called waters of hydration., compounds that contain specific ratios of loosely bound water molecules, called waters of hydrationThe loosely bound water molecules in hydrate compounds. These waters of hydration can often be removed by simply heating the compound.. Waters of hydration can often be removed simply by heating. For example, calcium dihydrogen phosphate can form a solid that contains one molecule of water per Ca(H2PO4)2 unit and is used as a leavening agent in the food industry to cause baked goods to rise. The empirical formula for the solid is Ca(H2PO4)2·H2O. In contrast, copper sulfate usually forms a blue solid that contains five waters of hydration per formula unit, with the empirical formula CuSO4·5H2O. When heated, all five water molecules are lost, giving a white solid with the empirical formula CuSO4 (Figure 4.3.1 ).
Figure 4.3.1 Loss of Water from a Hydrate with Heating. When blue CuSO4·5H2O is heated, two molecules of water are lost at 30°C, two more at 110°C, and the last at 250°C to give white CuSO4.
Compounds that differ only in the numbers of waters of hydration can have very different properties. For example, CaSO4·½H2O is plaster of Paris, which was often used to make sturdy casts for broken arms or legs, whereas CaSO4·2H2O is the less dense, flakier gypsum, a mineral used in drywall panels for home construction. When a cast would set, a mixture of plaster of Paris and water crystallized to give solid CaSO4·2H2O. Similar processes are used in the setting of cement and concrete.
Summary
An empirical formula gives the relative numbers of atoms of the elements in a compound, reduced to the lowest whole numbers. The formula unit is the absolute grouping represented by the empirical formula of a compound, either ionic or covalent. Empirical formulas are particularly useful for describing the composition of ionic compounds, which do not contain readily identifiable molecules. Some ionic compounds occur as hydrates, which contain specific ratios of loosely bound water molecules called waters of hydration.
Key Takeaway
- The composition of a compound is represented by an empirical or molecular formula, each consisting of at least one formula unit.
Conceptual Problems
-
What are the differences and similarities between a polyatomic ion and a molecule?
-
Classify each compound as ionic or covalent.
- Zn3(PO4)2
- C6H5CO2H
- K2Cr2O7
- CH3CH2SH
- NH4Br
- CCl2F2
-
Classify each compound as ionic or covalent. Which are organic compounds and which are inorganic compounds?
- CH3CH2CO2H
- CaCl2
- Y(NO3)3
- H2S
- NaC2H3O2
-
Generally, one cannot determine the molecular formula directly from an empirical formula. What other information is needed?
-
Give two pieces of information that we obtain from a structural formula that we cannot obtain from an empirical formula.
-
The formulas of alcohols are often written as ROH rather than as empirical formulas. For example, methanol is generally written as CH3OH rather than CH4O. Explain why the ROH notation is preferred.
-
The compound dimethyl sulfide has the empirical formula C2H6S and the structural formula CH3SCH3. What information do we obtain from the structural formula that we do not get from the empirical formula? Write the condensed structural formula for the compound.
-
What is the correct formula for magnesium hydroxide—MgOH2 or Mg(OH)2? Why?
-
Magnesium cyanide is written as Mg(CN)2, not MgCN2. Why?
-
Does a given hydrate always contain the same number of waters of hydration?
Answer
-
The structural formula gives us the connectivity of the atoms in the molecule or ion, as well as a schematic representation of their arrangement in space. Empirical formulas tell us only the ratios of the atoms present. The condensed structural formula of dimethylsulfide is (CH3)2S.
Numerical Problems
-
Write the formula for each compound.
- magnesium sulfate, which has 1 magnesium atom, 4 oxygen atoms, and 1 sulfur atom
- ethylene glycol (antifreeze), which has 6 hydrogen atoms, 2 carbon atoms, and 2 oxygen atoms
- acetic acid, which has 2 oxygen atoms, 2 carbon atoms, and 4 hydrogen atoms
- potassium chlorate, which has 1 chlorine atom, 1 potassium atom, and 3 oxygen atoms
- sodium hypochlorite pentahydrate, which has 1 chlorine atom, 1 sodium atom, 6 oxygen atoms, and 10 hydrogen atoms
-
Write the formula for each compound.
- cadmium acetate, which has 1 cadmium atom, 4 oxygen atoms, 4 carbon atoms, and 6 hydrogen atoms
- barium cyanide, which has 1 barium atom, 2 carbon atoms, and 2 nitrogen atoms
- iron(III) phosphate dihydrate, which has 1 iron atom, 1 phosphorus atom, 6 oxygen atoms, and 4 hydrogen atoms
- manganese(II) nitrate hexahydrate, which has 1 manganese atom, 12 hydrogen atoms, 12 oxygen atoms, and 2 nitrogen atoms
- silver phosphate, which has 1 phosphorus atom, 3 silver atoms, and 4 oxygen atoms
-
Complete the following table by filling in the formula for the ionic compound formed by each cation-anion pair.
Ion K+ Fe3+ NH4+ Ba2+ Cl− KCl SO42− PO43− NO3− OH− -
Write the empirical formula for the binary compound formed by the most common monatomic ions formed by each pair of elements.
- zinc and sulfur
- barium and iodine
- magnesium and chlorine
- silicon and oxygen
- sodium and sulfur
-
Write the empirical formula for the binary compound formed by the most common monatomic ions formed by each pair of elements.
- lithium and nitrogen
- cesium and chlorine
- germanium and oxygen
- rubidium and sulfur
- arsenic and sodium
-
Write the empirical formula for each compound.
- Na2S2O4
- B2H6
- C6H12O6
- P4O10
- KMnO4
-
Write the empirical formula for each compound.
- Al2Cl6
- K2Cr2O7
- C2H4
- (NH2)2CNH
- CH3COOH
- Draw the Lewis structures for
a. OH−
b. CN−
c. NO3−
d. SO42-−
Answers
-
- MgSO4
- C2H6O2
- C2H4O2
- KClO3
- NaOCl·5H2O
-
Ion K + Fe 3+ NH 4 + Ba 2+ Cl − KCl FeCl3 NH4Cl BaCl2 SO 4 2− K2SO4 Fe2(SO4)3 (NH4)2SO4 BaSO4 PO 4 3− K3PO4 FePO4 (NH4)3PO4 Ba3(PO4)2 NO 3 − KNO3 Fe(NO3)3 NH4NO3 Ba(NO3)2 OH − KOH Fe(OH)3 NH4OH Ba(OH)2 -
- Li3N
- CsCl
- GeO2
- Rb2S
- Na3As
-
- AlCl3
- K2Cr2O7
- CH2
- CH5N3
- CH2O
Contributors
- Anonymous
Modified by Joshua Halpern
Copper Hydrate Heating Video from kgreenchemistryonline.com on YouTube



