1: Solution Concentrations
- Page ID
- 155044
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In the laboratory, in your body, and in the outside environment, the majority of chemical reactions take place in solutions. Macroscopically a solution is defined as a homogeneous mixture of two or more substances, that is, a mixture which appears to be uniform throughout. On the microscopic scale a solution involves the random arrangement of one kind of atom or molecule with respect to another.
There are a number of reasons why solutions are so often encountered both in nature and in the laboratory. The most common type of solution involves a liquid solvent which dissolves a solid solute. (The term solvent usually refers to the substance present in greatest amount. There may be more than one solute dissolved in it.) Because a liquid adopts the shape of its container but does not expand to fill all space available to it, liquid solutions are convenient to handle. You can easily pour them from one container to another, and their volumes are readily measured using graduated cylinders, pipets, burets, volumetric flasks, or other laboratory glass-ware. Moreover, atoms or molecules of solids dissolved in a liquid are close together but still able to move past one another. They contact each other more frequently than if two solids were placed next to each other. This “intimacy” in liquid solutions often facilitates chemical reactions.

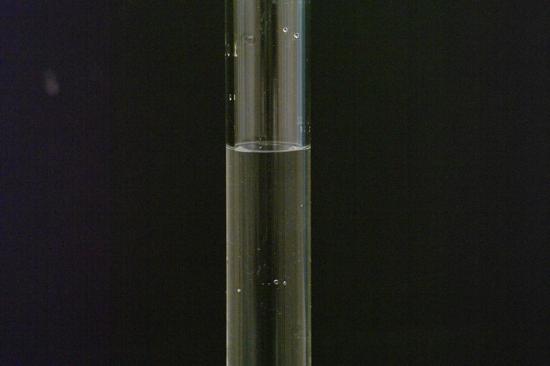
Pictured above is a familiar solution: salt water. In this solution, water serves as the solvent, dissolving the solute, salt.
Since solutions offer a convenient medium for carrying out chemical reactions, it is often necessary to know how much of one solution will react with a given quantity of another. Examples in other sections have shown that the amount of substance is the quantity which determines how much of one material will react with another. The ease with which solution volumes may be measured suggests that it would be very convenient to know the amount of substance dissolved per unit volume of solution. Then by measuring a certain volume of solution, we would also be measuring a certain amount of substance.
One measure of the concentration c of a solute in a solution is often called molarity, but it is probably better to call it "the concentration in molar units" or "molar concentration" (keeping the parameter concentration, and its unit, M for molar distinct). The molar concentration is the amount of the substance per unit volume (L or dm3) of solution(not solvent):
\[\text{Concentration of solute, M}=\frac{\text{amount of solute, mol}}{\text{volume of solution, L}}\]
\[c_{\text{solute, M}}~=~\frac{n_{\text{solute, mol}}}{V_{\text{solution,L}}} \label{2}\]
The units moles per liter (mol liter–1) or moles per cubic decimeter (mol dm–3) are used to express molar concentration. They are equivalent (since 1 dm–3 = 1 liter).
If a pure substance is soluble in water, it is easy to prepare a solution of known concentration. A container with a sample of the substance is weighed accurately, and an appropriate mass of sample is poured through a funnel into a volumetric flask, as shown in the figure. The container is then reweighed. Any solid adhering to the funnel is rinsed into the flask, and water is added until the flask is about three-quarters full. After swirling the flask to dissolve the solid, water is added carefully until the bottom of the meniscus coincides with the calibration mark on the neck of the flash. This process is shown in detail in Figure \(\PageIndex{1}\) :
|
a) a weight boat is zeroed on the balance. |
b) 58.441g of NaCl (~1 mole) is measured. |
|
c) The NaCl is quantitatively added to water. |
d) Mixing the solution dissolves NaCl. |
|
e) The solution is added to volumetric flask by funnel. |
f) Water is added to up the neck of the volumetric flask. |
|
g) A dropper is used to dilute to the 1 liter line. |
h) The meniscus reaches the mark. |
Example \(\PageIndex{1}\): Concentration
A solution of KI was prepared as described above. The initial mass of the container plus KI was 43.2874 g, and the final mass after pouring was 30.1544 g. The volume of the flask was 250.00 ml. What is the concentration of the solution?
Solution: The concentration can be calculated by dividing the amount of solute by the volume of solution [Eq. \(\ref{2}\)]:
\[ c_{\text{KI}} = \frac{n_{\text{KI, mol}}}{V_{\text{solution, L}}}\]
We obtain nKI from the mass of KI added to the flask:
\[ m_{\text{KI}} = 43.2874 - 30.1544 \text{ g} = 13.1330 \text{ g} \]
\[ n_{\text{KI}} = 13.1330 \text{ g} \times \frac{\text{1 mol}}{\text{166}\text{.00 g}} = 7.9115 \times 10^{-2} \text{ mol} \]
The volume of solution is 250.00 ml, or
\[ V_{\text{solution}} = 250.00 \text{cm}^{3} \times \frac{\text{1 dm}^{\text{3}}}{\text{10}^{\text{3}}\text{ cm}^{\text{3}}} = 2.5000 \times 10^{-1} \text{dm}^{3} \]
Thus
\[c_{\text{KI}}=\frac{n_{\text{KI}}}{V_{\text{solution}}}=\frac{\text{7}\text{.9115}\times \text{10}^{\text{-2}}\text{ mol }}{\text{2}\text{.50 }\times \text{10}^{\text{-1}}\text{ dm}^{\text{3}}}=\text{3}\text{.1645 }\times 10^{^{\text{-1}}}\text{mol dm}^{\text{-3}}\]
Note that the definition of concentration is entirely analogous to the definitions of density, molar mass, and stoichiometric ratio that we have previously encountered. Concentration will serve as a conversion factor relating the volume of solution to the amount of dissolved solute.
\[\text{Volume of solution}\overset{concentration}{\longleftrightarrow}\text{amount of solute} ~~~~~~~~~~~~~ V\overset{c}{\longleftrightarrow}n\]
Because the volume of a liquid can be measured quickly and easily, concentration is a much-used quantity. The next two examples show how this conversion factor may be applied to commonly encountered solutions in which water is the solvent (aqueous solutions).
Example \(\PageIndex{2}\) : Amount of HCl
An aqueous solution of HCl [represented or written HCl(aq)] has a concentration of 0.1396 mol dm–3. If 24.71 cm3 (24.71 ml) of this solution is delivered from a buret, what amount of HCl has been delivered?
Solution
Using concentration as a conversion factor, we have
\[V \text{ } \rightarrow {c} \text{ } n \]
\[n_{\text{HCl}}=\text{24}\text{.71 cm}^{\text{3}}\times \frac{\text{0}\text{.1396 mol}}{\text{1 dm}^{\text{3}}}\]
The volume units will cancel if we supply a unity factor to convert cubic centimeters to cubic decimeters:
\[ \begin{align} n_{\text{HCl}} & =\text{24} \text{.71 cm}^{\text{3}} \times \frac{\text{0} \text{.1396 mol}}{\text{1 dm}^{\text{3}}} \times ( \frac{\text{1 dm}}{\text{10 cm}} )^{\text{3}} \\ & =\text{24}\text{.71 cm}^{\text{3}}\times \frac{\text{0}\text{.1396 mol}}{\text{1 dm}^{\text{3}}}\times \frac{\text{1 dm}^{\text{3}}}{\text{10}^{\text{3}}\text{ cm}^{\text{3}}} \\ & = 0.003 450 \text{ mol} \end{align}\]
The concentration units of moles per cubic decimeter are often abbreviated M, pronounced molar. That is, a 0.1-M (one-tenth molar) solution contains 0.1 mol solute per cubic decimeter of solution. This abbreviation is very convenient for labeling laboratory bottles and for writing textbook problems; however, when doing calculations, it is difficult to see that
\[\text{1 dm}^{\text{3}}\times \text{1 }\text{M}=\text{1mol}\]
Therefore we recommend that you always write the units in full when doing any calculations involving solution concentrations. Also, it is sometimes easier to use the unit liter, which is equivalent to cubic deciliters:
\[\text{1 dm}^{\text{3}}\times \text{1 }\dfrac{\text{mol}}{\text{dm}^{\text{3}}}=\text{1mol}\]
\[\text{1 L}\times \text{1 }\dfrac{\text{mol}}{\text{L}}=\text{1mol}\]
Problems such as Example \(\PageIndex{2}\) are easier for some persons to solve if the solution concentration is expressed in millimoles per cubic centimeter (mmol cm–3) or millimoles per ml (1 ml = 1 cm–3) instead of moles per cubic decimeter. Since the SI prefix m means 10–3, 1 mmol = 10–3 mol, and
\[\text{1 M} ~ = ~ \dfrac{\text{1 mol}}{\text{1 dm}^{\text{3}}} ~ \times ~ \dfrac{\text{1 dm}^{\text{3}}}{\text{1 L}} ~ = ~ \dfrac{\text{1 mol}}{\text{L}}\]
\[\text{1 M} ~ = ~ \dfrac{\text{1 mol}}{\text{L}} ~ \times ~ \dfrac{\text{10}^{\text{-3}}\text{ L}}{\text{1 ml}} ~ \times ~ \dfrac{\text{1 mmol}}{\text{10}^{\text{-3}}\text{ mol}} ~ = ~ \dfrac{\text{1 mmol}}{\text{1 ml}}\]
\[\text{1 M} ~ = ~ \dfrac{\text{1 mol}}{\text{1 dm}^{\text{3}}} \times \dfrac{\text{1 dm}^{\text{3}}}{\text{10}^{\text{3}}\text{ cm}^{\text{3}}} \times \dfrac{\text{1 mmol}}{\text{10}^{\text{-3}}\text{ mol}} ~ = ~ \dfrac{\text{1 mmol}}{\text{1 cm}^{\text{3}}} \]
Thus a concentration of 0.1396 mol dm–3 (0.1396 M) can also be expressed as 0.1396 mmol cm–3, 0.1396 mol/L or 0.1396 mmol/mL. Expressing the concentration this way is very convenient when dealing with laboratory glassware calibrated in milliliters or cubic centimeters.
Example \(\PageIndex{3}\): Mass of NaOH
Exactly 25.0 ml NaOH solution whose concentration is 0.0974 M was delivered from a pipet.
- What amount of NaOH was present?
- What mass of NaOH would remain if all the water evaporated?
Solution:
a) Since 0.0974 M means 0.0974 mol dm–3, or 0.0974 mmol cm–3, we choose the latter, more convenient quantity as a conversion factor:
\[n_{\text{NaOH}}=\text{25}\text{.0 cm}^{\text{3}}\times \frac{\text{0}\text{.0974 mol}}{\text{1 cm}^{\text{3}}}=\text{2}\text{.44 mmol}=\text{2}\text{.44}\times 10^{\text{-3}}\text{ mol}\]
b) Using molar mass, we obtain
\[m_{\text{NaOH}}=\text{2}\text{.44}\times 10^{\text{-3}}\text{ mol}\times \frac{\text{40}\text{.01 g}}{\text{1 mol}}=9.\text{76}\times 10^{\text{-2}}\text{g}\]
The symbols \(n_{\ce{NaOH}}\) and \(m_{\ce{NaOH}}\) refer to the amount and mass of the solute \(\ce{NaOH}\), respectively. They do not refer to the solution. If we wanted to specify the mass of aqueous \(\ce{NaOH}\) solution, the symbol \(m_{\ce{NaOH(aq)}}\) could be used.
Contributors
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.