Learning Objective
- List the different types of cancer drugs and their mechanism of action.
Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents) as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent (which almost always involves combinations of drugs), or it may aim to prolong life or to reduce symptoms (palliative chemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called medical oncology.
When fighting cancer, the entire population of neoplastic cells must be eradicated in order to obtain desired results. The concept of "total cell-kill" applies to chemotherapy as it does to other means of treatment: total excision of the tumor is necessary for surgical care, and complete destruction of all cancer cells is required for a cure with radiation therapy.
The available anticancer drugs have distinct mechanisms of action which may vary in their effects on different types of normal and cancer cells. A single "cure" for cancer has proved elusive since there is not a single type of cancer but as many as 100 different types of cancer. In addition, there are very few demonstrable biochemical differences between cancerous cells and normal cells. For this reason the effectiveness of many anticancer drugs is limited by their toxicity to normal rapidly growing cells in the intestinal and bone marrow areas. A final problem is that cancerous cells which are initially suppressed by a specific drug may develop a resistance to that drug. For this reason cancer chemotherapy may consist of using several drugs in combination for varying lengths of time.
Introduction
Chemotherapy drugs, are sometimes feared because of a patient's concern about toxic effects. Their role is to slow and hopefully halt the growth and spread of a cancer. There are three goals associated with the use of the most commonly-used anticancer agents.
- Damage the DNA of the affected cancer cells.
- Inhibit the synthesis of new DNA strands to stop the cell from replicating, because the replication of the cell is what allows the tumor to grow.
- Stop mitosis or the actual splitting of the original cell into two new cells. Stopping mitosis stops cell division (replication) of the cancer and may ultimately halt the progression of the cancer.
Unfortunately, the majority of drugs currently on the market are not specific, which leads to the many common side effects associated with cancer chemotherapy. Because the common approach of all chemotherapy is to decrease the growth rate (cell division) of the cancer cells, the side effects are seen in bodily systems that naturally have a rapid turnover of cells including skin, hair, gastrointestinal, and bone marrow. These healthy, normal cells, also end up damaged by the chemotherapy program.
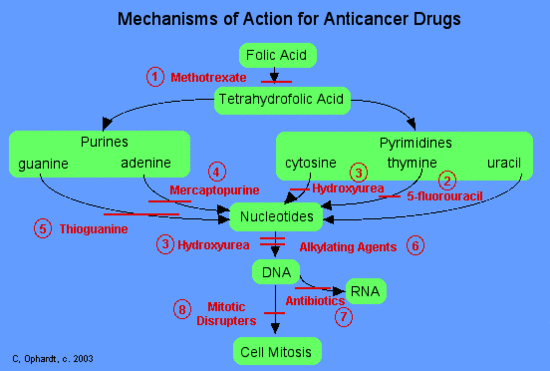
Categories of Chemotherapy Drugs
In general, chemotherapy agents can be divided into three main categories based on their mechanism of action as shown in the table below.
Table \(\PageIndex{1}\) Chemotherapy Drugs and Their Mechanism of Action
| Category |
Specific Mode of Action |
Examples |
|
Stop the synthesis of pre DNA molecule building blocks
"Antimetabolites"
|
These agents work in a number of different ways. DNA building blocks are folic acid, heterocyclic bases, and nucleotides, which are made naturally within cells. All of these agents work to block some step in the formation of nucleotides or deoxyribonucleotides (necessary for making DNA). When these steps are blocked, the nucleotides, which arethe building blocks of DNA and RNA, can not be synthesized. Thus the cells can not replicate because they cannot make DNA without the nucleotides. |
1) methotrexate (Abitrexate®),2) fluorouracil (Adrucil®), 3) hydroxyurea (Hydrea®), 4) mercaptopurine (Purinethol®) and 5) thioguanine. |
|
Directly damage the DNA in the nucleus of the cell
"Alkylating agents, antibiotics, topoisomerase inhibitors and intercalating agents."
|
These agents chemically damage DNA and RNA. They disrupt replication of the DNA and either totally halt replication or cause the manufacture of nonsense DNA or RNA (i.e. the new DNA or RNA does not code for anything useful). |
5) cisplatin (Platinol®) and 7) antibiotics - daunorubicin (Cerubidine®), doxorubicin (Adriamycin®), and etoposide (VePesid®). |
|
Effect the synthesis or breakdown of the mitotic spindles
"Mitotic disrupters"
|
Mitotic spindles serve as molecular railroads with "North and South Poles" in the cell when a cell starts to divide itself into two new cells. These spindles are very important because they help to split the newly copied DNA such that a copy goes to each of the two new cells during cell division. These drugs disrupt the formation of these spindles and therefore interrupt cell division. |
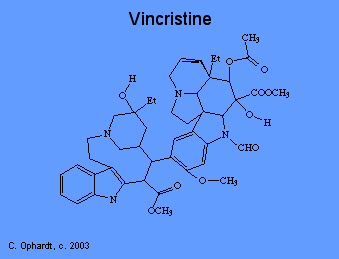
8) mitotic disrupters include: Vinblastine (Velban®), Vincristine (Oncovin®) and Pacitaxel (Taxol®). |
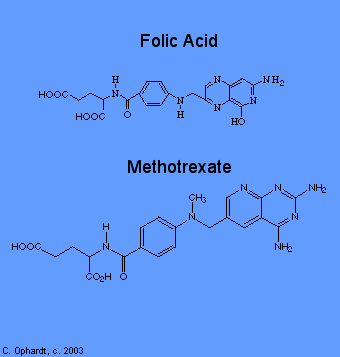
Methotrexate
Methotrexate inhibits folic acid reductase which is responsible for the conversion of folic acid to tetrahydrofolic acid. At two stages in the biosynthesis of purines(adenine and guanine) and at one stage in the synthesis of pyrimidines (thymine, cytosine, and uracil), one-carbon transfer reactions occur which require specific coenzymes synthesized in the cell from tetrahydrofolic acid.
Tetrahydrofolic acid itself is synthesized in the cell from folic acid with the help of an enzyme, folic acid reductase. Methotrexate looks a lot like folic acid to the enzyme, so it binds to it thinking that it is folic acid. In fact, methotrexate looks so good to the enzyme that it binds to it quite strongly and inhibits the enzyme. Thus, DNA synthesis cannot proceed because the coenzymes needed for one-carbon transfer reactions are not produced from tetrahydrofolic acid because there is no tetrahydrofolic acid. Again, without DNA, no cell division.
5-Fluorouracil
5-Fluorouracil (5-FU; Adrucil®, Fluorouracil, Efudex®, Fluoroplex®) is an effective pyrimidine antimetabolite. Fluorouracil is synthesized into the nucleotide, 5-fluoro-2-deoxyuridine. This product acts as an antimetabolite by inhibiting the synthesis of 2-deoxythymidine because the carbon - fluorine bond is extremely stable and prevents the addition of a methyl group in the 5-position. The failure to synthesize the thymidine nucleotide results in little or no production of DNA. Two other similar drugs include: gemcitabine (Gemzar®) and arabinosylcytosine (araC). They all work through similar mechanisms.

Hydroxyurea
Hydroxyurea blocks an enzyme which converts the cytosine nucleotide into the deoxy derivative. In addition, DNA synthesis is further inhibited because hydroxyurea blocks the incorporation of the thymidine nucleotide into the DNA strand.
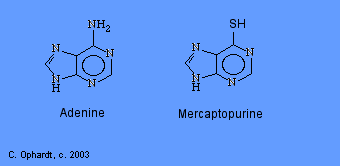
Mercaptopurine
Mercaptopurine, a chemical analog of the purine adenine, inhibits the biosynthesis of adenine nucleotides by acting as an antimetabolite. In the body, 6-MP is converted to the corresponding ribonucleotide. 6-MP ribonucleotide is a potent inhibitor of the conversion of a compound called inosinic acid to adenine Without adenine, DNA cannot be synthesized. 6-MP also works by being incorporated into nucleic acids as thioguanosine, rendering the resulting nucleic acids (DNA, RNA) unable to direct proper protein synthesis.
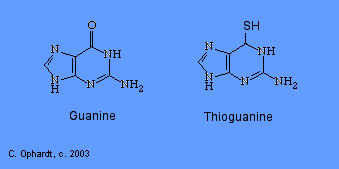
Thioguanine
Thioguanine is an antimetabolite in the synthesis of guanine nucleotides.
Alkylating Agents, Topoisomerase Inhibitors, Antibiotics, and Intercalating agents
Alkylating Agents
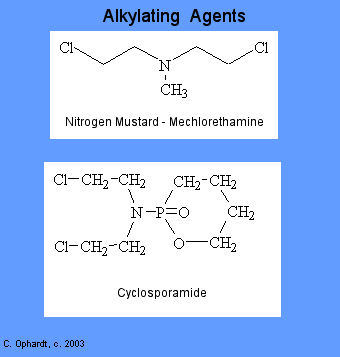
Alkylating agents involve reactions with guanine in DNA. These drugs add methyl or other alkyl groups onto molecules where they do not belong. This in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA.
There are six groups of alkylating agents: nitrogen mustards; ethylenimes; alkylsulfonates; triazenes; piperazines; and nitrosureas. Cyclosporamide is a classical example of the role of the host metabolism in the activation of an alkylating agent and is one or the most widely used agents of this class. It was hoped that the cancer cells might posses enzymes capable of accomplishing the cleavage, thus resulting in the selective production of an ated nitrogen mustard in the malignant cells. Compare the top and bottom structures in the graphic on the left.
Topoisomerase Inhibitors
Topoisomerase inhibitors are drugs that affect the activity of two enzymes: topoisomerase I and topoisomerase II. Inhibition of topoisomerase I or II interferes with both replication and transcription.
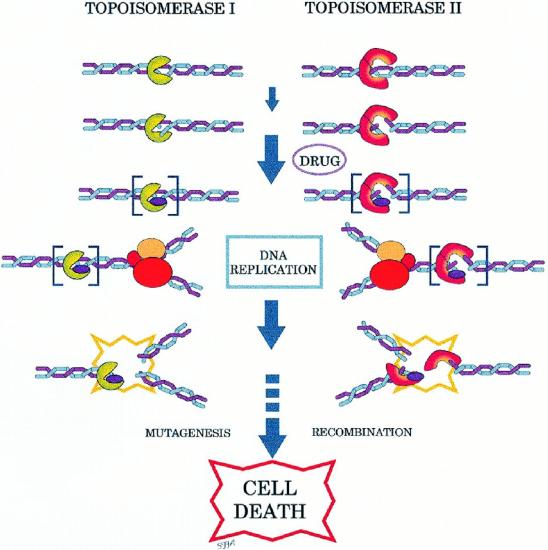
Two topoisomerase I inhibitors, irinotecan and topotecan, are semi-synthetically derived from camptothecin, which is obtained from the Chinese ornamental tree Camptotheca acuminata. Drugs that target topoisomerase II can be divided into two groups. The topoisomerase II inhibitors include etoposide, doxorubicin, mitoxantrone , teniposide, novobiocin, merbarone, and aclarubicin.
Antibiotics
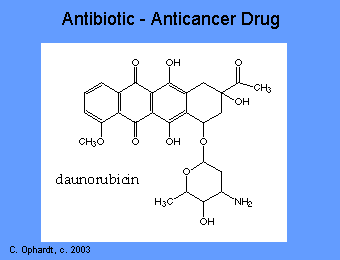
A number of antibiotics such as anthracyclines, dactinomycin, bleomycin, adriamycin, mithramycin, bind to DNA and inactivate it. Thus the synthesis of RNA is prevented. General properties of these drugs include: interaction with DNA in a variety of different ways including intercalation (squeezing between the base pairs), DNA strand breakage and inhibition with the enzyme topoisomerase II. Most of these compounds have been isolated from natural sources and antibiotics. However, they lack the specificity of the antimicrobial antibiotics and thus produce significant toxicity.
The anthracyclines are among the most important antitumor drugs available. Doxorubicin is widely used for the treatment of several solid tumors while daunorubicin and idarubicin are used exclusively for the treatment of leukemia. These agents have a number of important effects including: intercalating (squeezing between the base pairs) with DNA affecting many functions of the DNA including DNA and RNA synthesis. Breakage of the DNA strand can also occur by inhibition of the enzyme topoisomerase II.
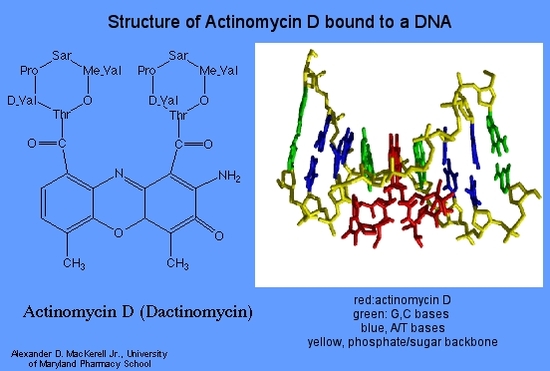
Dactinomycin (Actinomycin D)
At low concentrations dactinomycin inhibits DNA directed RNA synthesis and at higher concentrations DNA synthesis is also inhibited. All types of RNA are affected, but ribosomal RNA is more sensitive. Dactinomycin binds to double stranded DNA , permitting RNA chain initiation but blocking chain elongation. Binding to the DNA depends on the presence of guanine.
Intercalating Agents
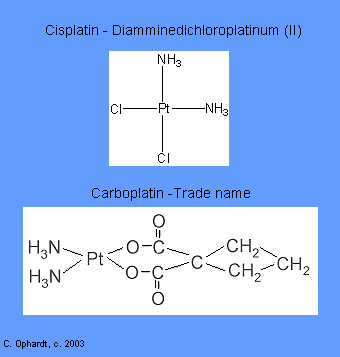
Intercalating agents wedge between bases along the DNA. The intercalated drug molecules affect the structure of the DNA, preventing polymerase and other DNA binding proteins from functioning properly. The result is prevention of DNA synthesis, inhibition of transcription and induction of mutations. Examples include: Carboplatin and Cisplatin.
Mitotic Disrupters
Mitotic Disrupters
Plant alkaloids like vincristine prevent cell division, or mitosis. There are several phases of mitosis, one of which is the metaphase. During metaphase, the cell pulls duplicated DNA chromosomes to either side of the parent cell in structures called "spindles". These spindles ensure that each new cell gets a full set of DNA. Spindles are microtubular fibers formed with the help of the protein "tubulin". Vincristine binds to tubulin, thus preventing the formation of spindles and cell division.
Taxol
Paclitaxel (taxol) was first isolated from the from the bark of the Pacific Yew (Taxus brevifolia). Docetaxel is a more potent analog that is produced semisynthetically. In contrast to other microtubule antagonists, taxol disrupts the equilibrium between free tubulin and mircrotubules by shifting it in the direction of assembly, rather than disassembly. As a result, taxol treatment causes both the stabilization of microtubules and the formation of abnormal bundles of microtubules. The net effect is still the disruption of mitosis.
Combination Chemotherapy
In 1965, a major breakthrough in cancer therapy occurred. James F. Holland, Emil Freireich, and Emil Frei hypothesized that cancer chemotherapy should follow the strategy of antibiotic therapy for tuberculosis with combinations of drugs, each with a different mechanism of action. Cancer cells could conceivably mutate to become resistant to a single agent, but by using different drugs concurrently it would be more difficult for the tumor to develop resistance to the combination. Holland, Freireich, and Frei simultaneously administered methotrexate (an antifolate), vincristine (a Vinca alkaloid), 6-mercaptopurine (6-MP) and prednisone — together referred to as the POMP regimen — and induced long-term remissions in children with acute lymphoblastic leukaemia (ALL). With incremental refinements of original regimens, using randomized clinical studies by St. Jude Children's Research Hospital, the Medical Research Council in the UK (UKALL protocols) and German Berlin-Frankfurt-Münster clinical trials group (ALL-BFM protocols), ALL in children has become a largely curable disease.
This approach was extended to the lymphomas in 1963 by Vincent T. DeVita and George Canellos at the NCI, who ultimately proved in the late 1960s that nitrogen mustard, vincristine, procarbazine and prednisone — known as the MOPP regimen — could cure patients with Hodgkin's and non-Hodgkin's lymphoma.
Currently, nearly all successful cancer chemotherapy regimens use this paradigm of multiple drugs given simultaneously, called combination chemotherapy or polychemotherapy.
Table \(\PageIndex{2}\) The Common Combination Chemotherapy Regimens
| Cancer type |
Drugs |
Acronym |
| Breast cancer |
Cyclophosphamide, methotrexate, 5-fluorouracil, vinorelbine |
CMF |
| Doxorubicin, cyclophosphamide |
AC |
| Hodgkin's lymphoma |
Docetaxel, doxorubicin, cyclophosphamide |
TAC |
| Doxorubicin, bleomycin, vinblastine, dacarbazine |
ABVD |
| Mustine, vincristine, procarbazine, prednisolone |
MOPP |
| Non-Hodgkin's lymphoma |
Cyclophosphamide, doxorubicin, vincristine, prednisolone |
CHOP |
| Germ cell tumor |
Bleomycin, etoposide, cisplatin |
BEP |
| Stomach cancer |
Epirubicin, cisplatin, 5-fluorouracil |
ECF |
| Epirubicin, cisplatin, capecitabine |
ECX |
| Bladder cancer |
Methotrexate, vincristine, doxorubicin, cisplatin |
MVAC |
| Lung cancer |
Cyclophosphamide, doxorubicin, vincristine, vinorelbine |
CAV |
| Colorectal cancer |
5-fluorouracil, folinic acid, oxaliplatin |
FOLFOX |
Summary
- Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents) as part of a standardized chemotherapy regimen.
- Chemo drugs can be classified into three main categories based on their mechanism of action namely:
- Stop the synthesis of pre-DNA molecule building blocks
- Directly damage the DNA in the nucleus of the cell
- Effect the synthesis or breakdown of the mitotic spindles.
- Currently, nearly all successful cancer chemotherapy regimens use this paradigm of multiple drugs given simultaneously, called combination chemotherapy or polychemotherapy.












