10.2: Pressure
- Page ID
- 55011
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- to describe and measure the pressure of a gas.
At the macroscopic level, a complete physical description of a sample of a gas requires four quantities:
- temperature (expressed in kelvins),
- volume (expressed in liters),
- amount (expressed in moles), and
- pressure (in atmospheres).
As we demonstrated below, these variables are not independent (i.e., they cannot be arbitrarily be varied). If we know the values of any three of these quantities, we can calculate the fourth and thereby obtain a full physical description of the gas. Temperature, volume, and amount have been discussed in previous chapters. We now discuss pressure and its units of measurement.
Units of Pressure
Any object, whether it is your computer, a person, or a sample of gas, exerts a force on any surface with which it comes in contact. The air in a balloon, for example, exerts a force against the interior surface of the balloon, and a liquid injected into a mold exerts a force against the interior surface of the mold, just as a chair exerts a force against the floor because of its mass and the effects of gravity. If the air in a balloon is heated, the increased kinetic energy of the gas eventually causes the balloon to burst because of the increased pressure (\(P\)) of the gas, the force (\(F\)) per unit area (\(A\)) of surface:
\[P=\dfrac{\rm Force}{\rm Area}=\dfrac{F}{A}\label{10.2.1} \]
Pressure is dependent on both the force exerted and the size of the area to which the force is applied. We know from Equation \(\ref{10.2.1}\) that applying the same force to a smaller area produces a higher pressure. When we use a hose to wash a car, for example, we can increase the pressure of the water by reducing the size of the opening of the hose with a thumb.
The units of pressure are derived from the units used to measure force and area. The SI unit for pressure, derived from the SI units for force (newtons) and area (square meters), is the newton per square meter (\(N/m^2\)), which is called the Pascal (Pa), after the French mathematician Blaise Pascal (1623–1662):
\[\rm 1 \;Pa=1\;N/m^2 \label{10.2.2} \]
Assuming a paperback book has a mass of 2.00 kg, a length of 27.0 cm, a width of 21.0 cm, and a thickness of 4.5 cm, what pressure does it exert on a surface if it is
- lying flat?
- standing on edge in a bookcase?
Given: mass and dimensions of object
Asked for: pressure
Strategy:
- Calculate the force exerted by the book and then compute the area that is in contact with a surface.
- Substitute these two values into Equation \(\ref{10.2.1}\) to find the pressure exerted on the surface in each orientation.
Solution:
The force exerted by the book does not depend on its orientation. Recall that the force exerted by an object is F = ma, where m is its mass and a is its acceleration. In Earth’s gravitational field, the acceleration is due to gravity (9.8067 m/s2 at Earth’s surface). In SI units, the force exerted by the book is therefore
\[F = ma = 2.00 \;\rm kg\times 9.8067 \dfrac{\rm m}{\rm s^2} = 19.6 \dfrac{\rm kg·m}{\rm s^2} = 19.6\;\rm N \nonumber \]
A We calculated the force as 19.6 N. When the book is lying flat, the area is
\[A=\rm0.270 \;m\times0.210 \;m= 0.0567 \;m^2. \nonumber \]
B The pressure exerted by the text lying flat is thus
\[P=\dfrac{F}{A}=\dfrac{19.6\;\rm N}{0.0567\;\rm m^2}=3.46\times10^2 \rm Pa \nonumber \]
A If the book is standing on its end, the force remains the same, but the area decreases:
\[\rm A=\rm21.0 \;cm\times4.5 \;cm = 0.210 \;m\times0.045 \;m = 9.5 \times 10^{−3} \;\rm m^2 \nonumber \]
B The pressure exerted by the text lying flat is thus
\[P=\dfrac{19.6\;\rm N}{9.5\times10^{-3}\;\rm m^2}=2.06\times10^3 \;\rm Pa \nonumber \]
What pressure does a 60.0 kg student exert on the floor
- when standing flat-footed in the laboratory in a pair of tennis shoes (the surface area of the soles is approximately 180 cm2)?
- as she steps heel-first onto a dance floor wearing high-heeled shoes (the area of the heel = 1.0 cm2)?
- Answer a
-
3.27 × 104 Pa
- Answer b
-
5.9 × 106 Pa
Barometric Pressure
Just as we exert pressure on a surface because of gravity, so does our atmosphere. We live at the bottom of an ocean of gases that becomes progressively less dense with increasing altitude. Approximately 99% of the mass of the atmosphere lies within 30 km of Earth’s surface (Figure \(\PageIndex{1}\)). Every point on Earth’s surface experiences a net pressure called barometric pressure. The pressure exerted by the atmosphere is considerable: a 1 m2 column, measured from sea level to the top of the atmosphere, has a mass of about 10,000 kg, which gives a pressure of about 101 kPa:

Barometric pressure can be measured using a barometer, a device invented in 1643 by one of Galileo’s students, Evangelista Torricelli (1608–1647). A barometer may be constructed from a long glass tube that is closed at one end. It is filled with mercury and placed upside down in a dish of mercury without allowing any air to enter the tube. Some of the mercury will run out of the tube, but a relatively tall column remains inside (Figure \(\PageIndex{2}\)). Why doesn’t all the mercury run out? Gravity is certainly exerting a downward force on the mercury in the tube, but it is opposed by the pressure of the atmosphere pushing down on the surface of the mercury in the dish, which has the net effect of pushing the mercury up into the tube. Because there is no air above the mercury inside the tube in a properly filled barometer (it contains a vacuum), there is no pressure pushing down on the column. Thus the mercury runs out of the tube until the pressure exerted by the mercury column itself exactly balances the pressure of the atmosphere. The pressure exerted by the mercury column can be expressed as:
\[\begin{align} P&=\dfrac{F}{A} \\[4pt] &= \dfrac{mg}{A} \\[4pt] &= \dfrac{\rho V\cdot g}{A} \\[4pt] &= \dfrac{ \rho \cdot Ah\cdot g}{A} \\[4pt] &= \rho gh \end{align} \nonumber \]
with
- \(g\) is the gravitational acceleration,
- \(m\) is the mass,
- \(\rho\) is the density,
- \(V\) is the volume,
- \(A\) is the bottom area, and
- \(h\) is height of the mercury column.
Under normal weather conditions at sea level, the two forces are balanced when the top of the mercury column is approximately 760 mm above the level of the mercury in the dish, as shown in Figure \(\PageIndex{2}\). This value varies with meteorological conditions and altitude. In Denver, Colorado, for example, at an elevation of about 1 mile, or 1609 m (5280 ft), the height of the mercury column is 630 mm rather than 760 mm.
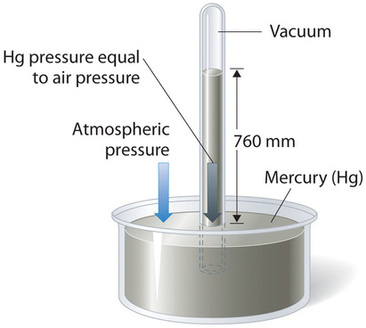
Mercury barometers have been used to measure barometric pressure for so long that they have their own unit for pressure: the millimeter of mercury (mmHg), often called the torr, after Torricelli. Standard barometric pressure is the barometric pressure required to support a column of mercury exactly 760 mm tall; this pressure is also referred to as 1 atmosphere (atm). These units are also related to the pascal:
\[\begin{align} \rm 1\; atm &= 760 \; mmHg \\[4pt] &= 760 \; torr \\[4pt] &= 1.01325 \times 10^5 \; Pa \\[4pt] &= 101.325 \; kPa\label{10.2.3} \end{align} \]
Thus a pressure of 1 atm equals 760 mmHg exactly.
We are so accustomed to living under this pressure that we never notice it. Instead, what we notice are changes in the pressure, such as when our ears pop in fast elevators in skyscrapers or in airplanes during rapid changes in altitude. We make use of barometric pressure in many ways. We can use a drinking straw because sucking on it removes air and thereby reduces the pressure inside the straw. The barometric pressure pushing down on the liquid in the glass then forces the liquid up the straw.
One of the authors visited Rocky Mountain National Park several years ago. After departing from an airport at sea level in the eastern United States, he arrived in Denver (altitude 5280 ft), rented a car, and drove to the top of the highway outside Estes Park (elevation 14,000 ft). He noticed that even slight exertion was very difficult at this altitude, where the barometric pressure is only 454 mmHg. Convert this pressure to
- atmospheres (atm).
- bar.
Given: pressure in millimeters of mercury
Asked for: pressure in atmospheres and bar
Strategy:
Use the conversion factors in Equation \(\ref{10.2.3}\) to convert from millimeters of mercury to atmospheres and kilopascals.
Solution:
From Equation \(\ref{10.2.3}\), we have 1 atm = 760 mmHg = 101.325 kPa. The pressure at 14,000 ft in atm is thus
\[ \begin{align} P &=\rm 454 \;mmHg\times\dfrac{1\;atm}{760\;mmHg} \\[4pt] &= 0.597\;atm \nonumber \end{align} \nonumber \]
\[ \begin{align} P&=\rm 0.597\;atm\times\dfrac{1.01325\;bar}{1\;atm}\\[4pt] &= 0.605\;bar \nonumber \end{align} \nonumber \]
Mt. Everest, at 29,028 ft above sea level, is the world’s tallest mountain. The normal barometric pressure at this altitude is about 0.308 atm. Convert this pressure to
- millimeters of mercury.
- bar.
- Answer a
-
234 mmHg;
- Answer b
-
0.312 bar
Manometers
Barometers measure barometric pressure, but manometers measure the pressures of samples of gases contained in an apparatus. The key feature of a manometer is a U-shaped tube containing mercury (or occasionally another nonvolatile liquid). A closed-end manometer is shown schematically in part (a) in Figure \(\PageIndex{3}\). When the bulb contains no gas (i.e., when its interior is a near vacuum), the heights of the two columns of mercury are the same because the space above the mercury on the left is a near vacuum (it contains only traces of mercury vapor). If a gas is released into the bulb on the right, it will exert a pressure on the mercury in the right column, and the two columns of mercury will no longer be the same height. The difference between the heights of the two columns is equal to the pressure of the gas.
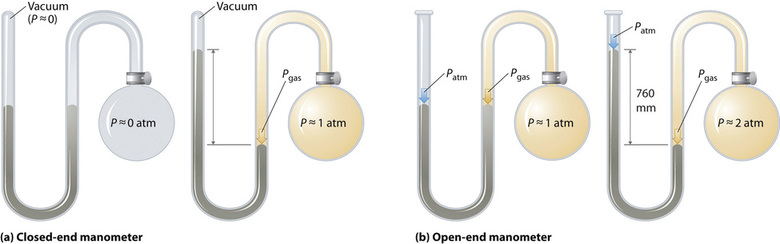
If the tube is open to the atmosphere instead of closed, as in the open-end manometer shown in part (b) in Figure \(\PageIndex{3}\), then the two columns of mercury have the same height only if the gas in the bulb has a pressure equal to the barometric pressure. If the gas in the bulb has a higher pressure, the mercury in the open tube will be forced up by the gas pushing down on the mercury in the other arm of the U-shaped tube. The pressure of the gas in the bulb is therefore the sum of the barometric pressure (measured with a barometer) and the difference in the heights of the two columns. If the gas in the bulb has a pressure less than that of the atmosphere, then the height of the mercury will be greater in the arm attached to the bulb. In this case, the pressure of the gas in the bulb is the barometric pressure minus the difference in the heights of the two columns.
Suppose you want to construct a closed-end manometer to measure gas pressures in the range 0.000–0.200 atm. Because of the toxicity of mercury, you decide to use water rather than mercury. How tall a column of water do you need? (The density of water is 1.00 g/cm3; the density of mercury is 13.53 g/cm3.)
Given: pressure range and densities of water and mercury
Asked for: column height
Strategy:
- Calculate the height of a column of mercury corresponding to 0.200 atm in millimeters of mercury. This is the height needed for a mercury-filled column.
- From the given densities, use a proportion to compute the height needed for a water-filled column.
Solution:
A In millimeters of mercury, a gas pressure of 0.200 atm is
\[P=\rm 0.200\;atm\times\dfrac{760\;mmHg}{1\;atm}=152\;mmHg \nonumber \]
Using a mercury manometer, you would need a mercury column at least 152 mm high.
B Because water is less dense than mercury, you need a taller column of water to achieve the same pressure as a given column of mercury. The height needed for a water-filled column corresponding to a pressure of 0.200 atm is proportional to the ratio of the density of mercury to the density of water
\[P=d_{\rm wat}gh_{\rm wat}=d_{\rm Hg}gh_{\rm Hg} \nonumber \]
\[h_{\rm wat}=h_{\rm Hg}\times\dfrac{d_{\rm Hg}}{g_{\rm wat}}=\rm152\;mm\times\dfrac{13.53\;g/cm^3}{1.00\;g/cm^3}=2070\;mm \nonumber \]
The answer makes sense: it takes a taller column of a less dense liquid to achieve the same pressure.
Suppose you want to design a barometer to measure barometric pressure in an environment that is always hotter than 30°C. To avoid using mercury, you decide to use gallium, which melts at 29.76°C; the density of liquid gallium at 25°C is 6.114 g/cm3. How tall a column of gallium do you need if P = 1.00 atm?
- Answer
-
1.68 m
The answer to Example \(\PageIndex{3}\) also tells us the maximum depth of a farmer’s well if a simple suction pump will be used to get the water out. The 1.00 atm corresponds to a column height of
A suction pump is just a more sophisticated version of a straw: it creates a vacuum above a liquid and relies on barometric pressure to force the liquid up a tube. If 1 atm pressure corresponds to a 10.3 m (33.8 ft) column of water, then it is physically impossible for barometric pressure to raise the water in a well higher than this. Until electric pumps were invented to push water mechanically from greater depths, this factor greatly limited where people could live because obtaining water from wells deeper than about 33 ft was difficult.
Summary
Pressure is defined as the force exerted per unit area; it can be measured using a barometer or manometer. Four quantities must be known for a complete physical description of a sample of a gas: temperature, volume, amount, and pressure. Pressure is force per unit area of surface; the SI unit for pressure is the pascal (Pa), defined as 1 newton per square meter (N/m2). The pressure exerted by an object is proportional to the force it exerts and inversely proportional to the area on which the force is exerted. The pressure exerted by Earth’s atmosphere, called barometric pressure, is about 101 kPa or 14.7 lb/in.2 at sea level. barometric pressure can be measured with a barometer, a closed, inverted tube filled with mercury. The height of the mercury column is proportional to barometric pressure, which is often reported in units of millimeters of mercury (mmHg), also called torr. Standard barometric pressure, the pressure required to support a column of mercury 760 mm tall, is yet another unit of pressure: 1 atmosphere (atm). A manometer is an apparatus used to measure the pressure of a sample of a gas.

