5.3: States of Matter: Review
- Page ID
- 213240
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define solid, liquid, and gas.
- Compare and contrast the proximities and mobilities of solid, liquid, and gaseous particles.
- Compare and contrast the volumes and shapes of chemicals that exist in the solid, liquid, and gaseous states of matter.
- Relate the volume and shape of a chemical substance to its particle-level characteristics.
As stated in Section 4.12, most substances can exist in one of four physical forms, or states of matter. The terms solid, \(\left( s \right)\), liquid, \(\left( l \right)\), and gas, \(\left( g \right)\), are all used to describe a single chemical. However, an aqueous, \(\left( aq \right)\), solution necessitates, by definition, the presence of the chemical that is being considered and the water in which that substance is dissolved. Because heat, which is the quantity that is being studied in the current chapter, is a measure of the amount of energy that is transferred between substances, the identity and amount of the material that is being studied must be clearly stated. Therefore, since a single chemical cannot be independently studied in an aqueous solution, substances that exist in this state of matter will not be discussed further in this chapter.
Solids
In the solid, \(\left( s \right)\), state of matter, the individual particles of a substance occupy fixed positions and must maintain direct contact with one another. As a result, a solid has a definite volume and a definite shape. Furthermore, since neither of these attributes is influenced by the vessel in which the substance is placed, both the volume and the shape of a solid are completely independent of its container.
Most solids are hard and brittle, meaning that they are likely to break under pressure. In contrast, some solids, such as elemental sodium, Na, which shown in the first image in Figure \(\PageIndex{1}\), are relatively soft and malleable. Sodium chloride, NaCl, which is shown in the second image in Figure \(\PageIndex{1}\), exists as a crystalline solid, meaning that its constituent cations and anions are arranged in a regular, three-dimensional array. In contrast, the particles that are present in some solids, such as rubbers and glasses, are not organized, and the corresponding substances are classified as amorphous, or "without form."

Liquids
In the liquid, \(\left( l \right)\), state of matter, the individual particles of a substance are able to move, but must still remain in direct contact with one another. As a result, a liquid has a definite volume, but does not have a definite shape. In other words, a substance that exists in its liquid state will occupy the same amount of space, regardless of the vessel in which it is poured. However, a liquid will mold itself into the shape of its container. For example, a liquid that is transferred into a cylindrical glass will take the form of a cylinder, but will reshape itself into a rectangle when poured into a rectangular pan. Therefore, the shape, but not the volume, of a liquid is directly dependent on the vessel in which it is placed.
Due to their close physical proximity, the individual molecules or atoms within a liquid are highly attracted to one another and, therefore, are not easily separated. This molecular-level resistance to separation, which is known as surface tension, causes liquid particles to adhere to one another and form "beads," or spherical droplets, when disturbed by an outside force. Both liquid water and mercury, which is the only known metal that exists in the liquid state of matter at room temperature, exhibit very high surface tensions, as evidenced by the "beading" that is shown in the first and second images, respectively, of Figure \(\PageIndex{2}\).

Gases
In the gaseous, \(\left( g \right)\), state of matter, the individual particles of a substance do not experience attractive forces and, therefore, are able to fully separate from one another and move randomly in three-dimensional space. As a result, gases, such as molecular chlorine, Cl2, have neither a defined volume nor a defined shape and will disperse until their constituent particles are uniformly distributed throughout the container in which they are placed, as shown below in Figure \(\PageIndex{3}\).

Because the particles that exist within a solid or a liquid must, by definition, maintain physical contact with one another, there is no "empty" space between these particles. As a result, the space that is occupied by a solid or a liquid chemical is equal to the volume of its constituent particles. Furthermore, the application of pressure does not impact the volume of a solid or liquid substance, as the direct physical contact of the corresponding constituent particles precludes these molecules or atoms from becoming any closer to one another. In contrast, gaseous chemicals exist as independent particles. As a result, the volume of a gaseous chemical is largely comprised of the "empty" spaces between its particles. Therefore, if pressure is applied to a gas, its constituent particles respond by shifting their positions to occupy these "empty" spaces. This spatial redistribution effectively decreases, or compresses, the overall volume of the gas by increasing the physical proximity of its particles. The ability to forcibly alter the volume of a gas has additional experimental implications, as will be discussed further in Chapter 6.
Comparing the States of Matter
This section has described the physical attractions that exist between solid, liquid, and gaseous particles. The effects of these molecular-level interactions can be represented using images, such as those that are shown below in Figure \(\PageIndex{4}\). In particular, these pictures illustrate the impact that attractive forces have on the spatial distribution of solid, liquid, and gaseous particles, respectively, relative to their container.
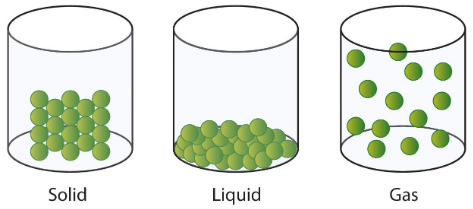
Finally, in order to more easily compare and contrast the qualitative aspects of solids, liquids, and gases, the content in the preceding paragraphs is summarized below in Table \(\PageIndex{1}\). Organizing information in this format highlights the correlations between the volume and shape of a substance and the proximity and mobility, respectively, of its constituent particles.
| Characteristic | Solid | Liquid | Gas |
|---|---|---|---|
| particle proximity | direct contact | direct contact | separated |
| volume | definite | definite | indefinite |
| particle mobility | immobile | mobile | mobile |
| shape | definite | indefinite | indefinite |

