5.7 Gases and Pressure
- Page ID
- 218386
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Skills to Develop
- Define the property of pressure
- Define and convert among the units of pressure measurements
- Describe the operation of common tools for measuring gas pressure
- Calculate pressure from manometer data
The earth’s atmosphere exerts a pressure, as does any other gas. Although we do not normally notice atmospheric pressure, we are sensitive to pressure changes—for example, when your ears “pop” during take-off and landing while flying, or when you dive underwater. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (Figure \(\PageIndex{1}\)). Although the force of each collision is very small, any surface of appreciable area experiences a large number of collisions in a short time, which can result in a high pressure. In fact, normal air pressure is strong enough to crush a metal container when not balanced by equal pressure from inside the container.
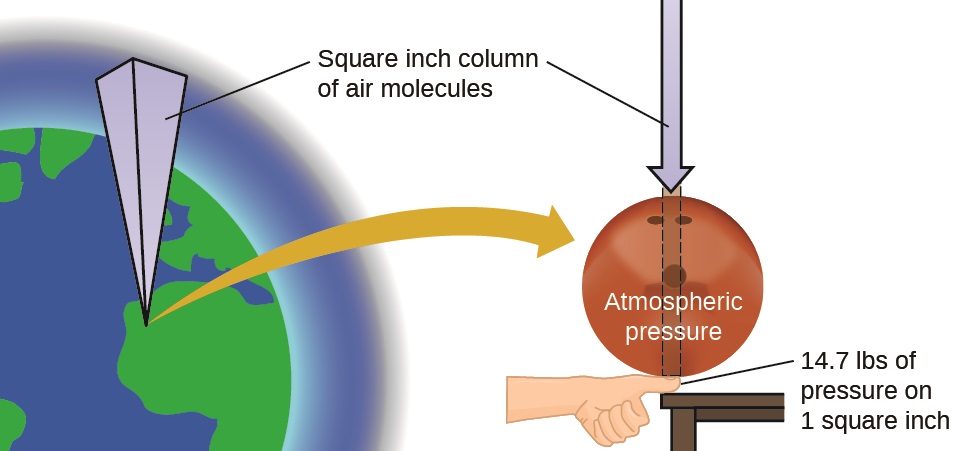
Figure \(\PageIndex{1}\): The atmosphere above us exerts a large pressure on objects at the surface of the earth, roughly equal to the weight of a bowling ball pressing on an area the size of a human thumbnail.
Atmospheric pressure is caused by the weight of the column of air molecules in the atmosphere above an object, such as the tanker car. At sea level, this pressure is roughly the same as that exerted by a full-grown African elephant standing on a doormat, or a typical bowling ball resting on your thumbnail. These may seem like huge amounts, and they are, but life on earth has evolved under such atmospheric pressure. If you actually perch a bowling ball on your thumbnail, the pressure experienced is twice the usual pressure, and the sensation is unpleasant.

A dramatic illustration of atmospheric pressure is provided in this brief video, which shows a railway tanker car imploding when its internal pressure is decreased.
Pressure is defined as the force exerted on a given area:
\[P=\dfrac{F}{A} \label{9.2.1}\]
Since pressure is directly proportional to force and inversely proportional to area (Equation \ref{9.2.1}), pressure can be increased either by either increasing the amount of force or by decreasing the area over which it is applied. Correspondingly, pressure can be decreased by either decreasing the force or increasing the area.
Let’s apply the definition of pressure (Equation \ref{9.2.1}) to determine which would be more likely to fall through thin ice in Figure \(\PageIndex{2}\).—the elephant or the figure skater?

Figure \(\PageIndex{2}\): Although (a) an elephant’s weight is large, creating a very large force on the ground, (b) the figure skater exerts a much higher pressure on the ice due to the small surface area of her skates. (credit a: modification of work by Guido da Rozze; credit b: modification of work by Ryosuke Yagi).
A large African elephant can weigh 7 tons, supported on four feet, each with a diameter of about 1.5 ft (footprint area of 250 in2), so the pressure exerted by each foot is about 14 lb/in2:
\[\mathrm{pressure\: per\: elephant\: foot=14,000\dfrac{lb}{elephant}×\dfrac{1\: elephant}{4\: feet}×\dfrac{1\: foot}{250\:in^2}=14\:lb/in^2} \label{9.2.2}\]
The figure skater weighs about 120 lbs, supported on two skate blades, each with an area of about 2 in2, so the pressure exerted by each blade is about 30 lb/in2:
\[\mathrm{pressure\: per\: skate\: blade=120\dfrac{lb}{skater}×\dfrac{1\: skater}{2\: blades}×\dfrac{1\: blade}{2\:in^2}=30\:lb/in^2} \label{9.2.3}\]
Even though the elephant is more than one hundred-times heavier than the skater, it exerts less than one-half of the pressure and would therefore be less likely to fall though thin ice. On the other hand, if the skater removes her skates and stands with bare feet (or regular footwear) on the ice, the larger area over which her weight is applied greatly reduces the pressure exerted:
The SI unit of pressure is the pascal (Pa), with 1 Pa = 1 N/m2, where N is the newton, a unit of force defined as 1 kg m/s2. One pascal is a small pressure; in many cases, it is more convenient to use units of kilopascal (1 kPa = 1000 Pa) or bar (1 bar = 100,000 Pa). In the United States, pressure is often measured in pounds of force on an area of one square inch—pounds per square inch (psi)—for example, in car tires. Pressure can also be measured using the unit atmosphere (atm), which originally represented the average sea level air pressure at the approximate latitude of Paris (45°). Table \(\PageIndex{1}\) provides some information on these and a few other common units for pressure measurements
| Unit Name and Abbreviation | Definition or Relation to Other Unit | Comment |
|---|---|---|
| pascal (Pa) | 1 Pa = 1 N/m2 | recommended IUPAC unit |
| kilopascal (kPa) | 1 kPa = 1000 Pa | |
| pounds per square inch (psi) | air pressure at sea level is ~14.7 psi | |
| atmosphere (atm) | 1 atm = 101,325 Pa | air pressure at sea level is ~1 atm |
| bar (bar, or b) | 1 bar = 100,000 Pa (exactly) | commonly used in meteorology |
| millibar (mbar, or mb) | 1000 mbar = 1 bar | |
| inches of mercury (in. Hg) | 1 in. Hg = 3386 Pa | used by aviation industry, also some weather reports |
| torr | \(\mathrm{1\: torr=\dfrac{1}{760}\:atm}\) | named after Evangelista Torricelli, inventor of the barometer |
| millimeters of mercury (mm Hg) | 1 mm Hg ~1 torr |
Example \(\PageIndex{1}\): Conversion of Pressure Units
The United States National Weather Service reports pressure in both inches of Hg and millibars. Convert a pressure of 29.2 in. Hg into:
- torr
- atm
- kPa
- mbar
Solution
This is a unit conversion problem. The relationships between the various pressure units are given in Table 9.2.1.
- \(\mathrm{29.2\cancel{in\: Hg}×\dfrac{25.4\cancel{mm}}{1\cancel{in}} ×\dfrac{1\: torr}{1\cancel{mm\: Hg}} =742\: torr}\)
- \(\mathrm{742\cancel{torr}×\dfrac{1\: atm}{760\cancel{torr}}=0.976\: atm}\)
- \(\mathrm{742\cancel{torr}×\dfrac{101.325\: kPa}{760\cancel{torr}}=98.9\: kPa}\)
- \(\mathrm{98.9\cancel{kPa}×\dfrac{1000\cancel{Pa}}{1\cancel{kPa}} \times \dfrac{1\cancel{bar}}{100,000\cancel{Pa}} \times\dfrac{1000\: mbar}{1\cancel{bar}}=989\: mbar}\)
Exercise \(\PageIndex{1}\)
A typical barometric pressure in Kansas City is 740 torr. What is this pressure in atmospheres, in millimeters of mercury, in kilopascals, and in bar?
- Answer
-
0.974 atm; 740 mm Hg; 98.7 kPa; 0.987 bar
We can measure atmospheric pressure, the force exerted by the atmosphere on the earth’s surface, with a barometer (Figure \(\PageIndex{3}\)). A barometer is a glass tube that is closed at one end, filled with a nonvolatile liquid such as mercury, and then inverted and immersed in a container of that liquid. The atmosphere exerts pressure on the liquid outside the tube, the column of liquid exerts pressure inside the tube, and the pressure at the liquid surface is the same inside and outside the tube. The height of the liquid in the tube is therefore proportional to the pressure exerted by the atmosphere.
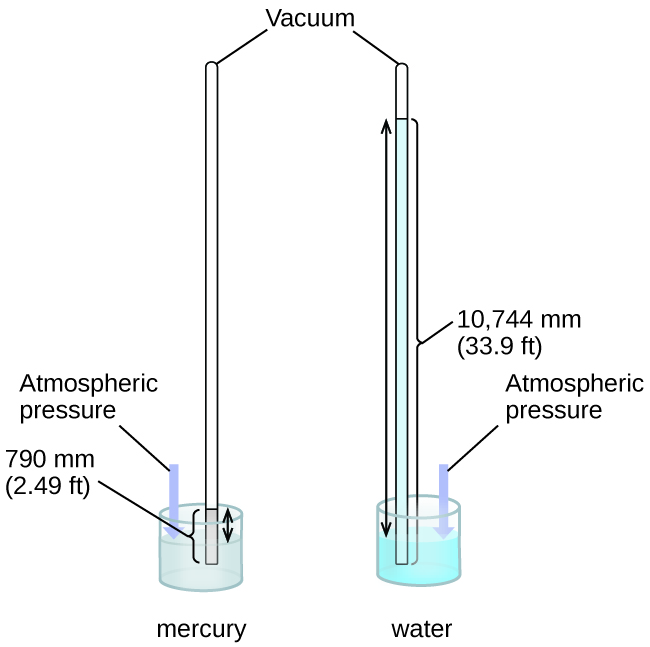
Figure \(\PageIndex{3}\): In a barometer, the height, h, of the column of liquid is used as a measurement of the air pressure. Using very dense liquid mercury (left) permits the construction of reasonably sized barometers, whereas using water (right) would require a barometer more than 30 feet tall.
If the liquid is water, normal atmospheric pressure will support a column of water over 10 meters high, which is rather inconvenient for making (and reading) a barometer. Because mercury (Hg) is about 13.6-times denser than water, a mercury barometer only needs to be \(\dfrac{1}{13.6}\) as tall as a water barometer—a more suitable size. Standard atmospheric pressure of 1 atm at sea level (101,325 Pa) corresponds to a column of mercury that is about 760 mm (29.92 in.) high. The torr was originally intended to be a unit equal to one millimeter of mercury, but it no longer corresponds exactly. The pressure exerted by a fluid due to gravity is known as hydrostatic pressure, p:
where
- \(h\) is the height of the fluid,
- \(ρ\) is the density of the fluid, and
- \(g\) is acceleration due to gravity.
Example \(\PageIndex{2}\): Calculation of Barometric Pressure
Show the calculation supporting the claim that atmospheric pressure near sea level corresponds to the pressure exerted by a column of mercury that is about 760 mm high. The density of mercury = \(13.6 \,g/cm^3\).
Solution
The hydrostatic pressure is given by Equation \ref{9.2.5}, with \(h = 760 \,mm\), \(ρ = 13.6\, g/cm^3\), and \(g = 9.81 \,m/s^2\). Plugging these values into the Equation \ref{9.2.5} and doing the necessary unit conversions will give us the value we seek. (Note: We are expecting to find a pressure of ~101,325 Pa:)
\[\mathrm{101,325\:\mathit{N}/m^2=101,325\:\dfrac{kg·m/s^2}{m^2}=101,325\:\dfrac{kg}{m·s^2}}\]
\[\begin {align*}
p&\mathrm{=\left(760\: mm×\dfrac{1\: m}{1000\: mm}\right)×\left(\dfrac{13.6\: g}{1\:cm^3}×\dfrac{1\: kg}{1000\: g}×\dfrac{( 100\: cm )^3}{( 1\: m )^3}\right)×\left(\dfrac{9.81\: m}{1\:s^2}\right)}\\
&\mathrm{=(0.760\: m)(13,600\:kg/m^3)(9.81\:m/s^2)=1.01 \times 10^5\:kg/ms^2=1.01×10^5\mathit{N}/m^2} \\ & \mathrm{=1.01×10^5\:Pa} \end {align*}\]
Exercise \(\PageIndex{2}\)
Calculate the height of a column of water at 25 °C that corresponds to normal atmospheric pressure. The density of water at this temperature is 1.0 g/cm3.
- Answer
-
10.3 m
A manometer is a device similar to a barometer that can be used to measure the pressure of a gas trapped in a container. A closed-end manometer is a U-shaped tube with one closed arm, one arm that connects to the gas to be measured, and a nonvolatile liquid (usually mercury) in between. As with a barometer, the distance between the liquid levels in the two arms of the tube (h in the diagram) is proportional to the pressure of the gas in the container. An open-end manometer (Figure \(\PageIndex{3}\)) is the same as a closed-end manometer, but one of its arms is open to the atmosphere. In this case, the distance between the liquid levels corresponds to the difference in pressure between the gas in the container and the atmosphere.
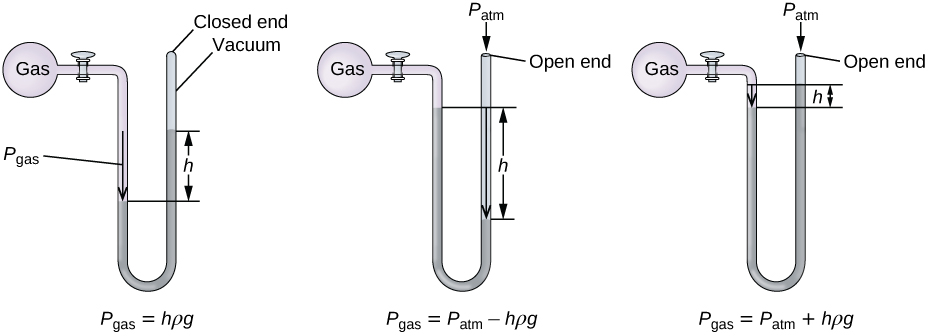
Figure \(\PageIndex{4}\): A manometer can be used to measure the pressure of a gas. The (difference in) height between the liquid levels (h) is a measure of the pressure. Mercury is usually used because of its large density.
Example \(\PageIndex{3}\): Calculation of Pressure Using an Open-End Manometer
The pressure of a sample of gas is measured at sea level with an open-end Hg (mercury) manometer, as shown below. Determine the pressure of the gas in:
- mm Hg
- atm
- kPa
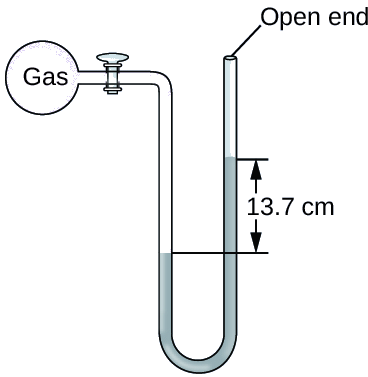
Solution
The pressure of the gas equals the hydrostatic pressure due to a column of mercury of height 13.7 cm plus the pressure of the atmosphere at sea level. (The pressure at the bottom horizontal line is equal on both sides of the tube. The pressure on the left is due to the gas and the pressure on the right is due to 13.7 cm of Hg plus atmospheric pressure.)
- In mm Hg, this is: 137 mm Hg + 760 mm Hg = 897 mm Hg
- \(\mathrm{897\cancel{mm Hg}×\dfrac{1\: atm}{760\cancel{mm Hg}}=1.18\: atm}\)
- \(\mathrm{1.18\cancel{atm}×\dfrac{101.325\: kPa}{1\cancel{atm}}=1.20×10^2\:kPa}\)
Exercise \(\PageIndex{3}\)
The pressure of a sample of gas is measured at sea level with an open-end Hg manometer, as shown below Determine the pressure of the gas in:
- mm Hg
- atm
- kPa
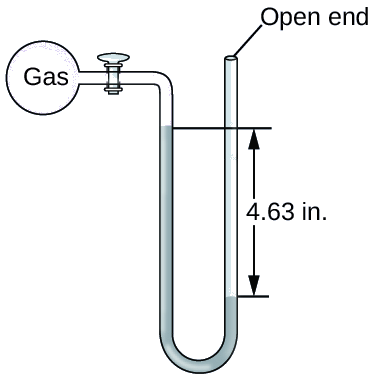
Answer a
-
642 mm Hg
- Answer b
-
0.845 atm
- Answer c
-
85.6 kPa
Meteorology, Climatology, and Atmospheric Science
Throughout the ages, people have observed clouds, winds, and precipitation, trying to discern patterns and make predictions: when it is best to plant and harvest; whether it is safe to set out on a sea voyage; and much more. We now face complex weather and atmosphere-related challenges that will have a major impact on our civilization and the ecosystem. Several different scientific disciplines use chemical principles to help us better understand weather, the atmosphere, and climate. These are meteorology, climatology, and atmospheric science. Meteorology is the study of the atmosphere, atmospheric phenomena, and atmospheric effects on earth’s weather. Meteorologists seek to understand and predict the weather in the short term, which can save lives and benefit the economy. Weather forecasts (Figure \(\PageIndex{5}\)) are the result of thousands of measurements of air pressure, temperature, and the like, which are compiled, modeled, and analyzed in weather centers worldwide.
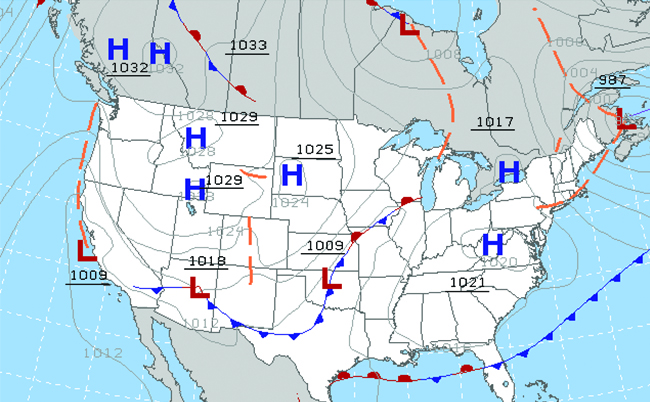
Figure \(\PageIndex{6}\): Meteorologists use weather maps to describe and predict weather. Regions of high (H) and low (L) pressure have large effects on weather conditions. The gray lines represent locations of constant pressure known as isobars. (credit: modification of work by National Oceanic and Atmospheric Administration)
In terms of weather, low-pressure systems occur when the earth’s surface atmospheric pressure is lower than the surrounding environment: Moist air rises and condenses, producing clouds. Movement of moisture and air within various weather fronts instigates most weather events.
The atmosphere is the gaseous layer that surrounds a planet. Earth’s atmosphere, which is roughly 100–125 km thick, consists of roughly 78.1% nitrogen and 21.0% oxygen, and can be subdivided further into the regions shown in Figure \(\PageIndex{7}\): the exosphere (furthest from earth, > 700 km above sea level), the thermosphere (80–700 km), the mesosphere (50–80 km), the stratosphere (second lowest level of our atmosphere, 12–50 km above sea level), and the troposphere (up to 12 km above sea level, roughly 80% of the earth’s atmosphere by mass and the layer where most weather events originate). As you go higher in the troposphere, air density and temperature both decrease.
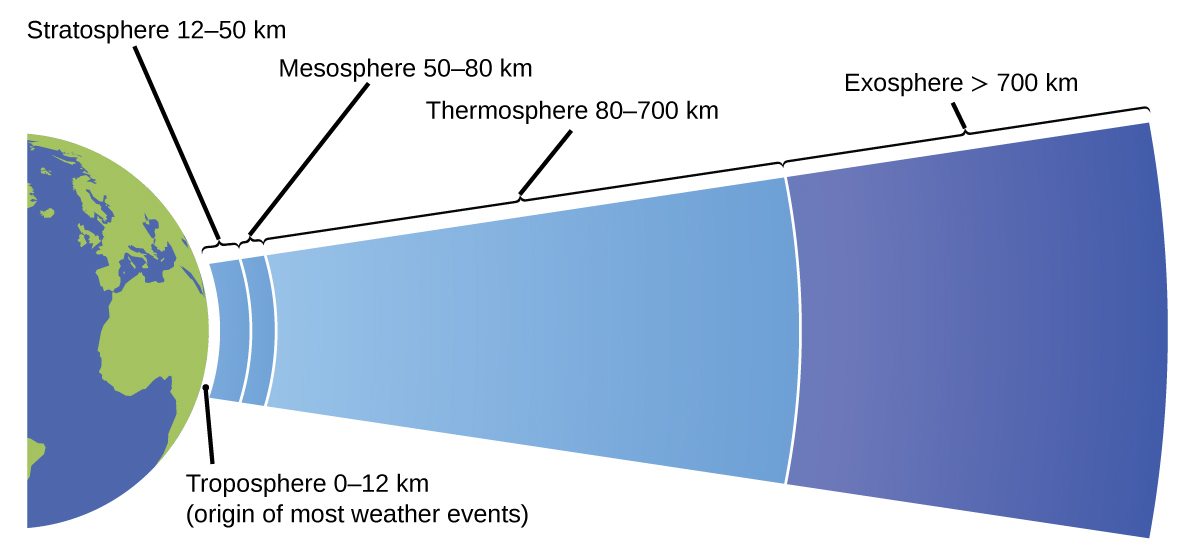
Figure \(\PageIndex{7}\): Earth’s atmosphere has five layers: the troposphere, the stratosphere, the mesosphere, the thermosphere, and the exosphere.
Climatology is the study of the climate, averaged weather conditions over long time periods, using atmospheric data. However, climatologists study patterns and effects that occur over decades, centuries, and millennia, rather than shorter time frames of hours, days, and weeks like meteorologists. Atmospheric science is an even broader field, combining meteorology, climatology, and other scientific disciplines that study the atmosphere.
A Practical Use of Air Pressure
A water well is an excavation or structure created in the ground by digging, driving, boring, or drilling to access groundwater in underground aquifers. The well water is often drawn by a pump (Figure \(\PageIndex{1}\)). Unfortunately, it impossible to pump water from very deep in the ground with just a surface pump. The key to understanding why is realizing that suction generated by the pump is not a force, but simply removing an opposing force to the force of air pressure which is already there. When you stick a pipe down a deep hole into a pool of water at the bottom of a well, air inside the pipe is pushing down on the water in the pipe, and air outside the pipe is pushing down on the water outside the pipe, which in turn pushes up on water inside the pipe - all is in a balance.
.jpg?revision=1&size=bestfit&width=451&height=422)
Figure \(\PageIndex{1}\): The manual water pump draws water up from a well by creating a vacuum that water rushes in to fill. Hand pump to pump water from a well in a village near Chennai in India. Image used with permission (CC BY 2.0; Sustainable Sanitation Alliance).
Let's say you remove the air inside the pipe. The water is pushed up the the same as it was before, but there is no counter acting force pushing the water down, so the water begins to rise inside the pipe (Figure \(\PageIndex{2}\)). So far so good, but the water stops rising at some height because the water is pulled down by gravity (i.e., the more water in the pipe, the more it weighs). Because the force of the air outside the pipe is not changing, eventually the weight of the water is equal to the air pressure outside the pipe. When this happens, the system is in balance again and water stops flowing.
Suction is not a force. Atmospheric pressure is the force that pushes the water up the pipe
Water is pumped from a well by creating a partial vacuum above the water by the pump. The amount of vacuum is equal to the weight of the column of water from the water table to the surface. Atmospheric pressure at sea level is 760 mm of mercury (\(1.01 \times 10^5 \,Pascals\)), which is equivalent to a 10.3-meter column of water. This is how deep water can be pumped from (with a surface pump; other pressurized pumps can go deeper).
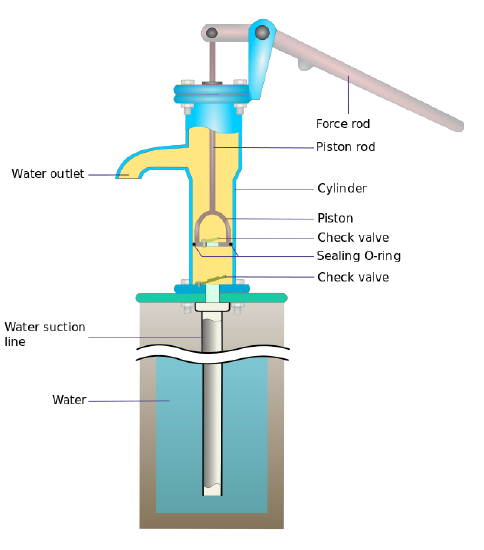
Figure \(\PageIndex{2}\): Cross section and details of a surface pump used in a well. Image used with permission (CC BY-SA 3.0; Manco Capac).
The Ideal Gas Law
An ideal gas is a mythical substance in which the gas particles have neither volume nor attractive intermolecular forces. Such a substance does not exist, but using the approximation creates a very simple mathematical description of a gas sample, the ideal gas law. The ideal gas law, PV = nRT, relates the four variables that describe any sample of a gas:
P is pressure, the force per unit area exerted by gas molecules as they bounce off of the surfaces that they hit. There are many units for measuring gas pressure. A unit you may have seen in a weather report is inches of mercury (in Hg), which are convenient units to use when measuring pressure with a mercury manometer. Another common unit is pounds per square inch (psi). However, we will use the unit of atmospheres (atm), an older pressure unit that was once defined as the pressure required to hold up a 760 mm column of Hg at 0oC. Colloquially, 1.00 atm is the pressure exerted by the atmosphere at sea level on an average day.
V is for volume, the amount of space that the gas sample is confined in. We will use the unit of Liters for our volume measurements.
n is for moles, the number of moles of gas particles in the sample.
T is for temperature, the property that allows us to determine the direction of heat flow and also gives us an idea of the kinetic energy of the gas molecules. We will use the Kelvin temperature scale for temperature, because we can not have any negative temperatures when describing gases. (Remember, the temperature in Kelvin = the temperature in Celsius plus 273.15o. K = 273.15 + oC.)
R is the gas constant, 0.08206 \(\dfrac{L·atm}{mole·K} \). This value can be obtained from the ratio of \(\dfrac {(22.4 \; L)(1.00 \; atm)}{(1.00 \; mole)(273.15 \; K)} \), employing the volume that 1.00 mole of an ideal gas occupies when held at 1.00 atm pressure and a temperature of 273.15 K, but it is true for any ideal gas under any set of conditions.
The ideal gas law is useful because if we know the values of any three of the four variables (P, V, T, and n) for a sample of gas, we can calculate the value of the fourth variable for that gaseous sample. The equation also allows us to predict the final state of a sample of a gas (i.e., its final temperature, pressure, volume, and amount) following any changes in conditions if the parameters (P, V, T, and n) are specified for an initial state. Some applications are illustrated in the following examples. The approach used throughout is always to start with the same equation—the ideal gas law—and then determine which quantities are given and which need to be calculated. Let’s begin with simple cases in which we are given three of the four parameters needed for a complete physical description of a gaseous sample.
Example \(\PageIndex{1}\)
A balloon with a volume of 31,150 L is to be filled with an ideal gas. If the temperature at ground level is 303 K, and the atmospheric pressure is 0.980 atm, how many moles of hydrogen gas will be needed to fill the balloon?
Given: volume, temperature, and pressure
Asked for: moles of gas
Strategy:
- Solve the ideal gas law for the unknown quantity, in this case n.
- Make sure that all quantities are given in units that are compatible with the units of the gas constant. If necessary, convert them to the appropriate units, insert them into the equation you have derived, and then calculate the number of moles of hydrogen gas needed.
Solution
\( n = \dfrac{PV}{RT} = \dfrac{(0.980 \; atm)(31150 \; L)}{(0.08206 \; \dfrac {L \; atm}{mole \; K})(303 \; K)} = 1.23 \times 10^3 moles \)
Exercise \(\PageIndex{1}\)
Suppose that an “empty” aerosol spray-paint can has a volume of 0.406 L and contains 0.0250 mol of a propellant gas such as CO2. What is the pressure of the gas at 25.0°C?
- Answer
-
1.51 L
Summary
Gases exert pressure, which is force per unit area. The pressure of a gas may be expressed in the SI unit of pascal or kilopascal, as well as in many other units including torr, atmosphere, and bar. Atmospheric pressure is measured using a barometer; other gas pressures can be measured using one of several types of manometers.
Key Equations
- \(P=\dfrac{F}{A}\)
- p = hρg
Glossary
- atmosphere (atm)
- unit of pressure; 1 atm = 101,325 Pa
- bar
- (bar or b) unit of pressure; 1 bar = 100,000 Pa
- barometer
- device used to measure atmospheric pressure
- hydrostatic pressure
- pressure exerted by a fluid due to gravity
- manometer
- device used to measure the pressure of a gas trapped in a container
- pascal (Pa)
- SI unit of pressure; 1 Pa = 1 N/m2
- pounds per square inch (psi)
- unit of pressure common in the US
- pressure
- force exerted per unit area
- torr
- unit of pressure; \(\mathrm{1\: torr=\dfrac{1}{760}\,atm}\)
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Modified by Tom Neils (Grand Rapids Community College)

