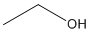4.1 Chemical Formulas
- Page ID
- 218254
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- To understand the definition and difference between empirical formulas and molecular formulas
- To understand how combustion analysis can be used to identify molecular formulas
Chemistry is the experimental and theoretical study of materials at both the macroscopic and microscopic levels. Understanding the relationship between properties and structures/bonding is a major aspect of these theoretical and experimental studies. A chemical formula is a format used to express the structure of compounds. The formula tells which elements and how many of each element are present in a compound. Formulas are written using the elemental symbol of each atom and a subscript to denote the number of elements.
Molecular formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced ratio of elements in a compound. If a compound's molecular formula cannot be reduced any more, then the empirical formula is the same as the molecular formula. Once the molar mass of the compound is known, the molecular formula can be calculated from the empirical formula.
Formulas on the Atomic and the Molar Level
A chemical formula tells us the relative ratios of different atoms in a compound. The ratios hold true on the molar level as well as the atomic level. Thus, H2O is composed of two atoms of hydrogen and 1 atom of oxygen. One molecule of water has a mass of 18.02 amus. Likewise, 1.000 mole of H2O molecules is composed of 2.000 moles of hydrogen and 1.000 mole of oxygen. The mass of 1.000 mole of water molecules has a mass of 18.02 grams.
Example \(\PageIndex{1}\): Acetone
An acetone molecule has the chemical formula of C3H6O.
Questions:
a) Is C3H6O an empirical formula, a molecular formula, or both?
b) How many H atoms are in one acetone molecule?
c) How many total atoms are in one acetone molecule?
d) What is the mass, in amus, of one acetone molecule?
e) What is the mass, in grams, of 1.000 mole of acetone molecules?
f) How many H atoms are in 1.000 mole of acetone molecules?
g) How many total atoms are in 1.000 mole of acetone molecules?
Solutions:
a) The formula is both an empirical formula (because it is the simplest ratio of elements) and also a molecular formula (because the formula tells the actual number of atoms of each element in one acetone molecule).
b) There are 6 H atoms in one acetone molecule.
c) There are 10 total atoms (6 H + 3 C + O) in one acetone molecule.
d) The mass of one acetone molecule: \(6 \; H \; atoms \times \dfrac{1.008 \; amus}{H \; atom} + 3 \; C \; atoms \times \dfrac{12.01 \; amus}{C \; atom} + 1 \; O \; atom \times \dfrac{16.00 \; amus}{O \; atom} = 58.08 amus\)
e) The mass of 1.000 mole of acetone molecules: \[6 \; moles \; H \; atoms \times \dfrac{1.008 \; grams}{1.000 \; moles \; H \; atoms} + 3 \; moles \; C \; atoms \times \dfrac{12.01 \; grams}{1.000 \; moles \; C \; atoms} + 1 \; mole \; O \; atoms \times \dfrac{16.00 \; grams}{1.000 \; mole \; O \; atoms} = 58.08 grams\]
f) H atoms in 1.00 mole of acetone molecules: \[1.000 \; mole \; acetone \; molecules \times \dfrac{6 \; moles \; H \; atoms}{1.000 \; mole \; acetone \; molecules} \times \dfrac{6.022 x 10^{23} \; H \; atoms}{1.000 \; moles \; H \; atoms} = 3.613 x 10^{24} H \; atoms \]
g) Total atoms in 1.000 moles of acetone molecules: \[1.000 \; mole \; acetone \; molecules \times \dfrac{10 \; moles \; atoms}{1.000 \; mole \; acetone \; molecules} \times \dfrac{6.022 x 10^{23} \; atoms}{1.000 \; mole \; atoms} = 6.022 x 10^{24} \; atoms \]
Exercise \(\PageIndex{1}\)
An certain molecule has the chemical formula of C8H8O4
Questions:
a) Is C8H8O4 an empirical formula, a molecular formula, or both?
b) How many H atoms are in one C8H8O4 molecule?
c) How many total atoms are in one C8H8O4 molecule?
d) What is the mass, in amus, of one C8H8O4 molecule?
e) What is the mass, in grams, of 1.000 mole of C8H8O4 molecules?
f) How many H atoms are in 1.000 mole of C8H8O4 molecules?
g) How many total atoms are in 1.000 mole of C8H8O4 molecules?
- Answer
-
a) The formula is a molecular formula, because it is not the simplest ratio of elements. The empirical formula is C2H2O1
b) There are 8 H atoms in one acetone molecule.
c) There are 20 total atoms (8 H + 8 C + 4 O) in one C8H8O4 molecule.
d) The mass of one C8H8O4 molecule: \(8 \; H \; atoms \times \dfrac{1.008 \; amus}{H \; atom} + 8 \; C \; atoms \times \dfrac{12.01 \; amus}{C \; atom} + 4 \; O \; atom \times \dfrac{16.00 \; amus}{O \; atom} = 168.1 amus\)
e) The mass of 1.000 mole of C8H8O4 molecules: \[8 \; moles \; H \; atoms \times \dfrac{1.008 \; grams}{1.000 \; moles \; H \; atoms} + 8 \; moles \; C \; atoms \times \dfrac{12.01 \; grams}{1.000 \; moles \; C \; atoms} + 4 \; mole \; O \; atoms \times \dfrac{16.00 \; grams}{1.000 \; mole \; O \; atoms} = 168.1 grams\]
f) H atoms in 1.00 mole of C8H8O4 molecules: \[1.000 \; mole \; C_8H_8O_4 \; molecules \times \dfrac{8 \; moles \; H \; atoms}{1.000 \; mole \; C_8H_8O_4 \; molecules} \times \dfrac{6.022 x 10^{23} \; H \; atoms}{1.000 \; moles \; H \; atoms} = 4.818 x 10^{24} H \; atoms \]
g) Total atoms in 1.000 moles of C8H8O4 molecules: \[1.000 \; mole \; C_8H_8O_4 \; molecules \times \dfrac{20 \; moles \; atoms}{1.000 \; mole \; C_8H_8O_4 \; molecules} \times \dfrac{6.022 x 10^{23} \; atoms}{1.000 \; mole \; atoms} = 1.204 x 10^{25} \; atoms \]
Structural Formulas
There are several ways to write and draw chemical formulas for covalently-bonded compounds, depending on how much information about the structure is needed.
Condensed Structural Formula
Condensed structural formulas show a bit more information about the bonding order of atoms in a molecule than a simple molecular formula. A condensed structural formula is written in a single line to save space and make it more convenient and faster to write out. Condensed structural formulas are also helpful when showing that a group of atoms is connected to a single atom in a compound. When this happens, parenthesis are used around the group of atoms to show they are together.
Ex. Condensed Structural Formula for ethanol: CH3CH2OH (molecular Formula: C2H6O).
Condensed Structural Formula for dimethyl ether: (CH3)2O (molecular formula: C2H6O).
Expanded Structural Formula
A structural formula displays the atoms of the molecule in the order they are bonded. It also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. Covalent bonds are shown using lines. The number of dashes indicate whether the bond is a single, double, or triple covalent bond. Structural formulas are helpful because they explain the properties and structure of the compound which condensed formulas cannot always represent.
Structural Formula for CH3CH2OH, ethanol
Line-Angle Formula
Because organic compounds can be complex, line-angle formulas are used to write carbon and hydrogen atoms more efficiently by omitting the symbol of H atoms attached to C atoms, and by replacing the C element symbols with the intersection of lines. Thus, a carbon atom is present wherever one line intersects another line. Hydrogen atoms are assumed to complete each of carbon's four bonds. All other atoms that are connected to carbon atoms are written out. Line angle formulas help show structure and order of the atoms in a compound making the advantages and disadvantages similar to structural formulas.
Line-Angle Formula for Ethanol:
Isomers
Understanding how atoms in a molecules are arranged and how they are bonded together is very important in giving the molecule its identity. Isomers are compounds in which two molecules have the same number of atoms, and thus the same molecular formula, but have completely different physical and chemical properties because of differences in structural formula.

Methylpropane and butane have the same molecular formula of C4H10, but are structurally different (methylpropane on the left, butane on the right).
Polymers
A polymer is formed when small molecules of identical structure, monomers, combine into a large cluster. The monomers are joined together by covalent bonds. When monomers repeat and bind, they form a polymer. While they can be comprised of natural or synthetic molecules, polymers often include plastics and rubber. When a molecule has more than one of these polymers, parenthesis are used to show that all the elements within the polymer are multiplied by the subscript outside of the parenthesis. The subscript (shown as n in the example below) denotes the number of monomers present in the macromolecule (or polymer).

Ethylene becomes the polymer polyethylene.
-
O
Contributors
- Modified by Tom Neils (Grand Rapids Community College)