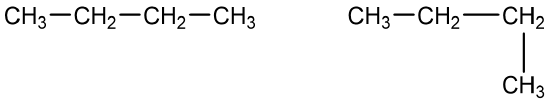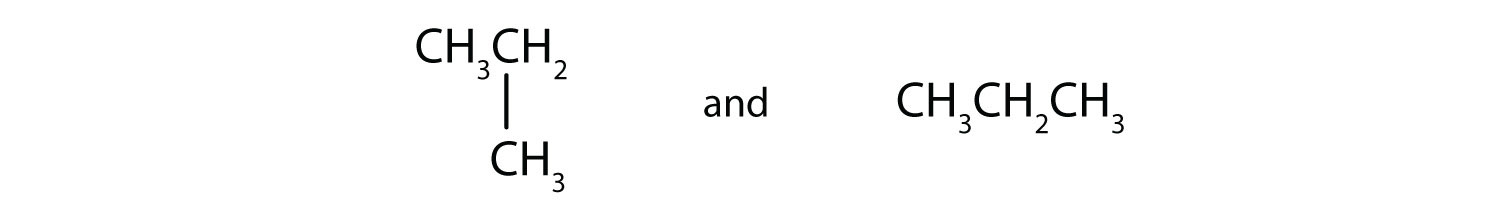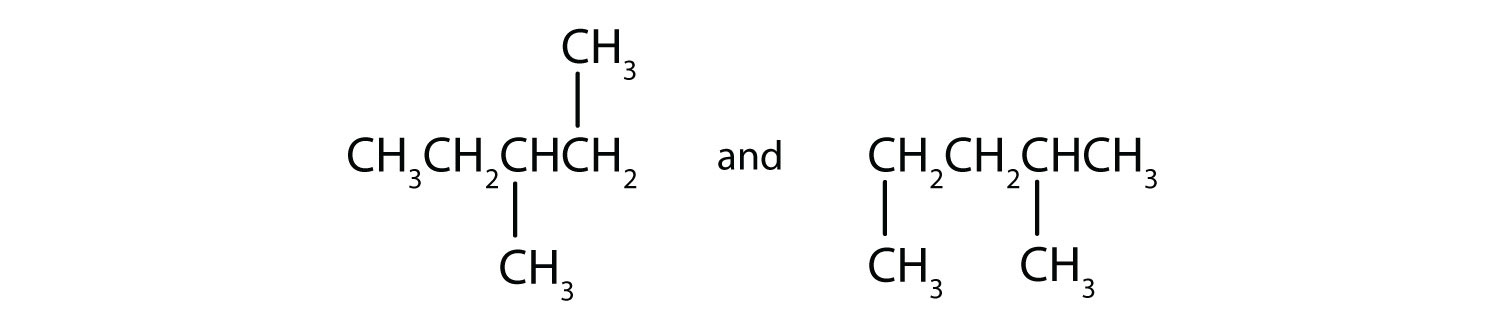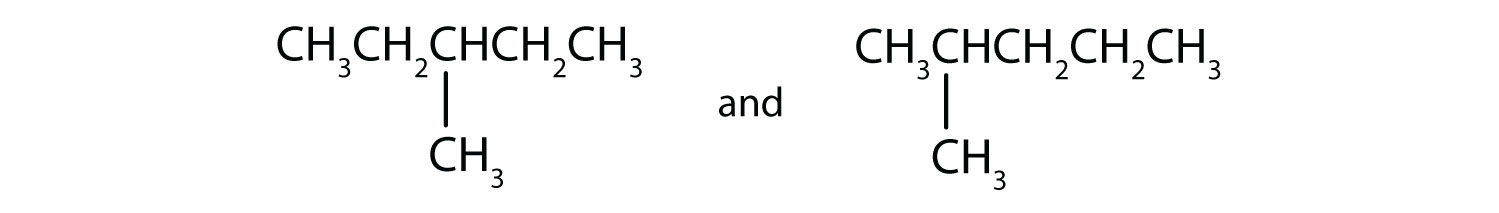1.E: CHEM 1151 Organic Review (Exercises)
- Page ID
- 338679
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)1.1 Organic Chemistry
Concept Review Exercises
- Classify each compound as organic or inorganic.
- C3H8O
- CaCl2
- Cr(NH3)3Cl3
- C30H48O3N
- Which compound is likely organic and which is likely inorganic?
- a flammable compound that boils at 80°C and is insoluble in water
- a compound that does not burn, melts at 630°C, and is soluble in water
- Which member of each pair has a higher melting point?
- CH3OH and NaOH
- CH3Cl and KCl
- CoCl2 and C2H6
- CH4 and LiH
Answers
-
- organic
- inorganic
- inorganic
- organic
-
- organic
- inorganic
-
- NaOH
- KCl
- CoCl2
- LiH
1.2 Structures of Organic Compounds
Concept Review Exercises
-
In alkanes, can there be a two-carbon branch off the second carbon atom of a four-carbon chain? Explain.
- A student is asked to write structural formulas for two different hydrocarbons having the molecular formula C5H12. She writes one formula with all five carbon atoms in a horizontal line. She then shows another with four carbon atoms in a line and a CH3 group extending down from the first carbon atom. Do these structural formulas represent different molecular formulas? Explain why or why not.
- A condensed structural formula for isohexane can be written as (CH3)2CHCH2CH2CH3. Draw the line-angle formula for isohexane.
- Give the structural formula for the compound represented by this line-angle formula:
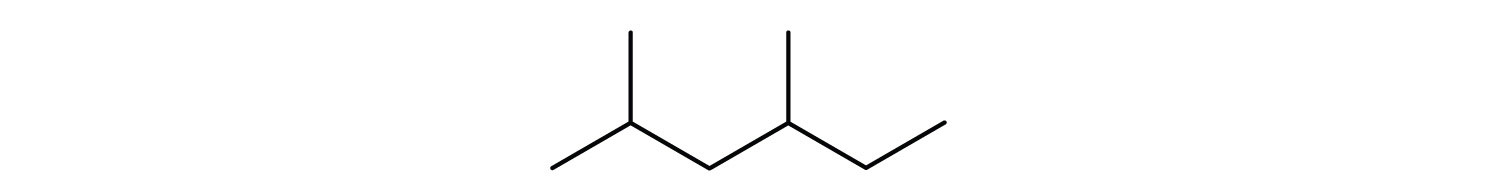
-
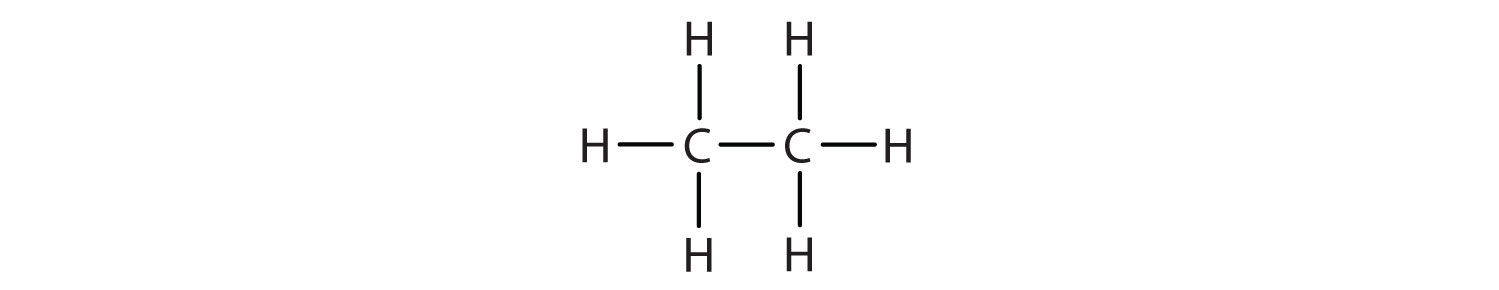
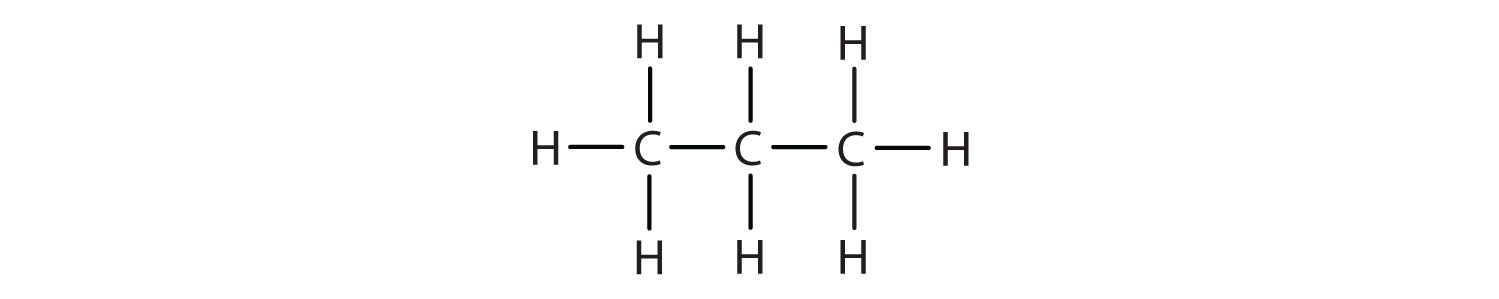
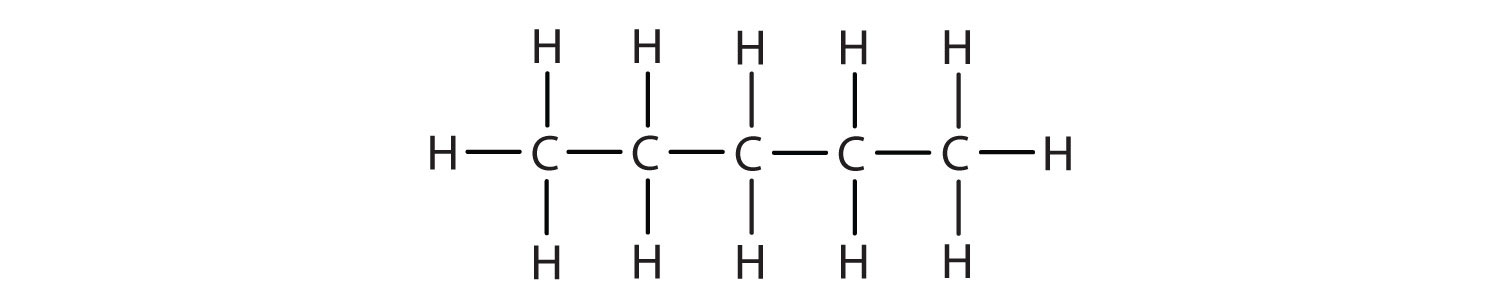
Write the condensed structural formula for each molecule.
Answers
- No; the branch would make the longest continuous chain of five carbon atoms.
- No; both are five-carbon continuous chains.
-

- CH3CH(CH3)CH2CH(CH3)CH2CH3
-
- CH3CH3
- CH3CH2CH3
- CH3CH2CH2CH2CH3
1.3 Branched Alkanes
Concept Review Exercises
-
Briefly identify the important distinctions between a straight-chain alkane and a branched-chain alkane.
-
How are butane and isobutane related? How do they differ?
-
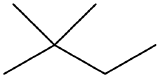
Draw a line-angle formula for the compound CH3CH2CH(CH3)CH2CH2CH3.
-
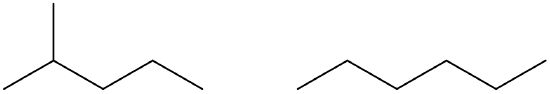
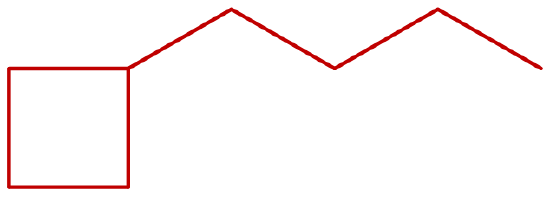
Indicate whether the structures in each set represent the same compound or isomers.
-
Answers
- Straight-chain alkanes and branched-chain alkanes have different properties as well as different structures. The names will also be different.
- Butane and isobutane have the same molecular formula, C4H10. However, the atoms are connected differently, so that have different names.
-
- isomers (left: branched alkane and right: straight-chain alkane)
- same compound (both are butane)
1.4 Alkane IUPAC Nomenclature
Concept Review Exercises
- What is a CH3 group called when it is attached to a chain of carbon atoms—a substituent or a functional group?
- How many carbon atoms are present in each molecule?
- 2,3-dimethylbutane
- 3-ethyl-2-methylheptane
- Draw the structures of the following compounds:
- 3-methylpentane
- 2,2,5-trimethylhexane
- 4-ethyl-3-methyloctane
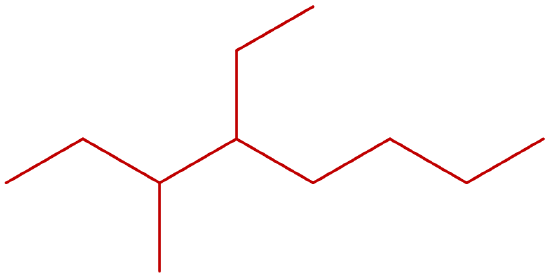
- Give the IUPAC name of the following compounds:
-
-
-
-
Answers
- substituent
-
- 6
- 10
- 11
-
-
-
- 2,2-dimethylbutane
- 3-ethyl-2-methylhexane
- 2,4-dimethylpentane
1.5 Halogenated Alkanes
Concept Review Exercises
- What is the IUPAC name for the HFC that has the formula CH2FCF3? (Hint: you must use a number to indicate the location of each substituent F atom)
- Write the condensed structural formula for each compound:
- ethyl bromide
- carbon tetrachloride
- Write the condensed structural formulas for the two isomers that have the molecular formula C3H7Br. Give the common name and the IUPAC name of each.
Answers
- 1,1,1,2-tetrafluoroethane
-
- CH3CH2Br
- CCl4
CH3CH2CH3Br common name: propyl bromide; IUPAC name: 1-bromopropane
CH3CH(Br)CH3 common name: isopropyl bromide; IUPAC name: 2-bromopropane
1.6 Cycloalkanes
Concept Review Exercises
- Draw the structure for each compound:
- methylcyclohexane
- butylcyclobutane
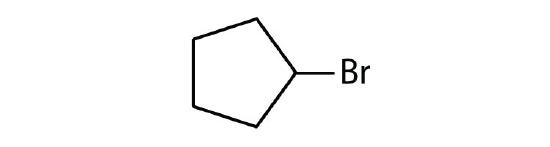
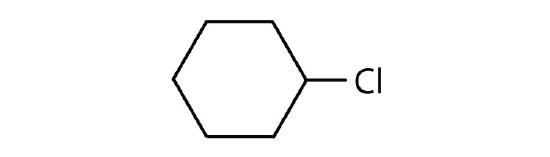
- Cycloalkyl groups can be derived from cycloalkanes in the same way that alkyl groups are derived from alkanes. These groups are named as cyclopropyl, cyclobutyl, and so on. Name each cycloalkyl halide.
-
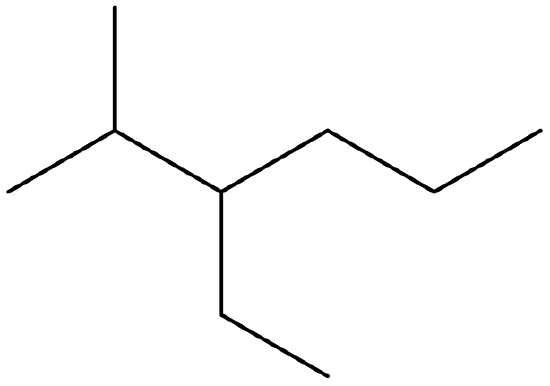
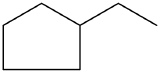
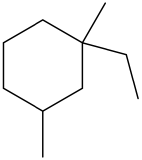
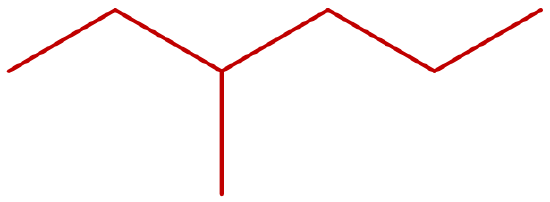
- Give the IUPAC name of the following molecule:
-
Answers
-
-
-
- cyclopentyl bromide
- cyclohexyl chloride
-
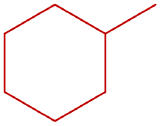
- ethylcyclopentane
- 1-ethyl-1,3-dimethylcyclohexane
Additional Exercises
-
You find an unlabeled jar containing a solid that melts at 48°C. It ignites readily and burns readily. The substance is insoluble in water and floats on the surface. Is the substance likely to be organic or inorganic?
-
Give the molecular formulas for methylcyclopentane, 2-methylpentane, and cyclohexane. Which are isomers?
-
What is wrong with each name? (Hint: first write the structure as if it were correct.) Give the correct name for each compound.
- 2-dimethylpropane
- 2,3,3-trimethylbutane
- 2,4-diethylpentane
- 3,4-dimethyl-5-propylhexane
-
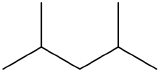
The following is the line formula for an alkane. Give the IUPAC name of the compound.
-
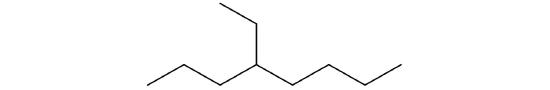
Draw the structures for the five isomeric hexanes (C6H14). Name each by the IUPAC system.
-
Indicate whether the structures in each set represent the same compound or isomers.
-
-
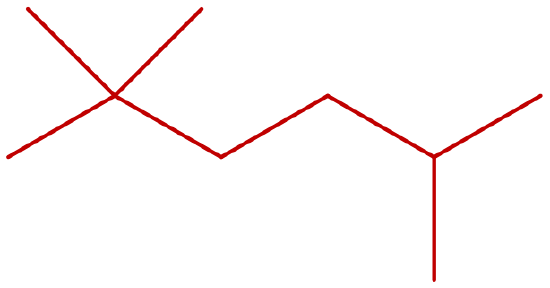
Consider the line-angle formulas shown here and answer the questions.
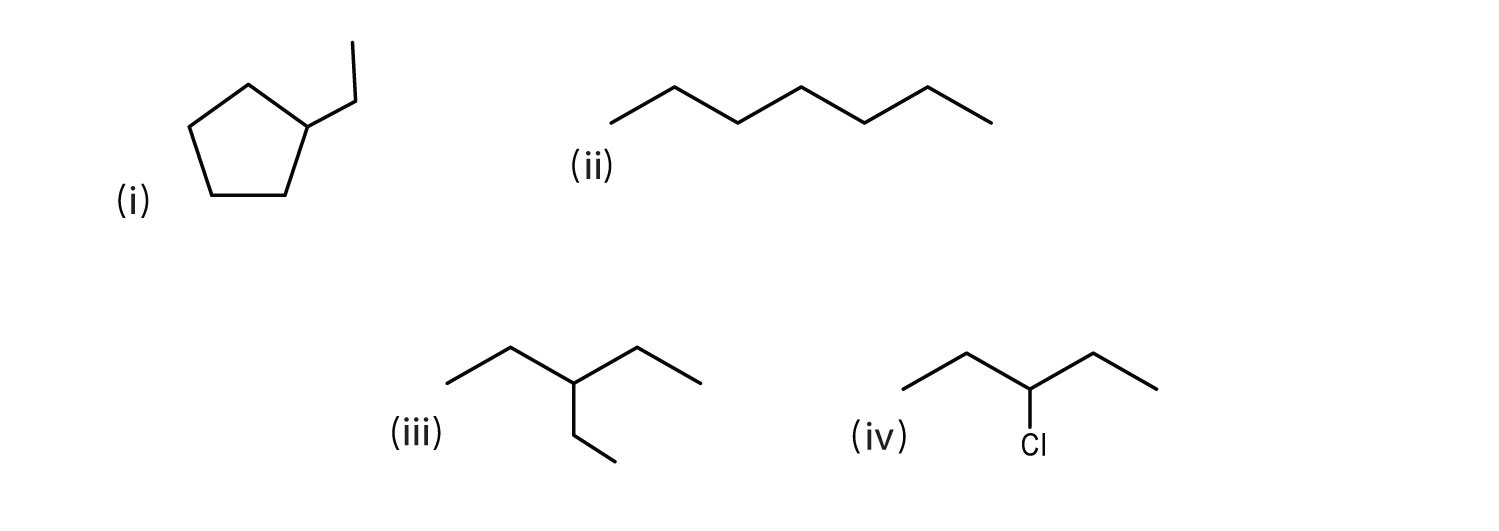
- Which pair of formulas represents isomers?
- Which formula represents an alkyl halide? Give the IUPAC name of the compound and write its condensed structural formula.
- Which formula represents a cycloalkane? Give the IUPAC name of the compound.
- What is the molecular formula of the compound represented by (i)?
Answers
-
organic
-
- Two numbers are needed to indicate two substituents; 2,2-dimethylpropane.
- The lowest possible numbers were not used; 2,2,3-trimethylbutane.
- An ethyl substituent is not possible on the second carbon atom; 3,5-dimethylheptane.
- A propyl substituent is not possible on the fifth carbon atom; 3,4,5-trimethyloctane.
-
 hexane
hexane 2-methylpentane
2-methylpentane 3-methylpentane
3-methylpentane 2,2-dimethylbutane
2,2-dimethylbutane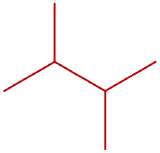 2,3-dimethylbutane
2,3-dimethylbutane
-
- ii and iii
- iv; 3-chloropentane; CH3CH2CHClCH2CH3
- i; ethylcyclopentane
- C7H14