5.1: The Discovery of Radiation
- Page ID
- 85159
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand the difference between induced and natural radioactivity
- Know which elements have unstable nuclei.
- Correlate the relationship between binding energy and stability.
From Section 3.3, we saw how J. J. Thomson discovered the electron by using cathode ray tubes (CRT). In November of 1895, a German physicist named Wilhelm Roentgen used this same technology to discover the X-ray. Roengten's ray originated from the CRT and reacted with a barium platinocyanide screen to fluoresce. After seeing this, Roentgen placed objects between this ray and the screen. He noted that the ray would still penetrate substances and leave an image on photographic film.
When naming this type of radiation, Roentgen reflected on his experiments. He could not confidently explain his observations. For this reason, he decided to call this new type of radiation the X-ray. Throughout his lifetime, he continued to explore this technology and even used his wife as a test subject (refer to Chapter 1 for her X-ray image). By never patenting this invention, Roengten freely shared the X-ray with other researchers and medical professionals. In 1901, he was awarded the Nobel Prize in Physics for his work with the x-ray.

As with any new technology, people looked for ways to apply the x-ray to day to day life. Thomas Edison, the inventor of the light bulb, thought the average person should have an x-ray machine in their home. He designed x-ray machines to be smaller and portable. Unfortunately, many of his technicians died from radiation poisoning.
In November 1896, an article entitled "the harmful effects of X-rays" was published in Nature. The witness was an X-ray demonstrator during the summer in London. He therefore paid for himself throughout the summer at the rate of several hours per day of exposure. He testified: "In the first two or three weeks I felt no inconvenience, but after a while appeared on the fingers of my right hand many dark spots which pierced under the skin, and gradually they became very painful; the rest of the skin was red and strongly inflamed My hand was so bad that I was constantly forced to bathe it in very cold water An ointment momentarily calm the pain but the epidermis had dried up had become hard and yellow like parchment and completely insensible, so I was not surprised when my hand began to peel. "
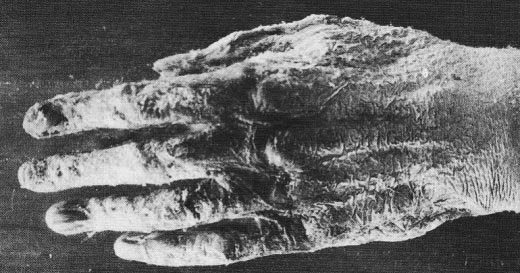
"Soon the skin and nails fall off and the fingers swelled, the pain remaining constant. I lost the skin of my right and left hands, and four of my nails have disappeared from the right hand and two left and three others were ready to fall off. During more than six weeks I was unable to hold anything in my right hand and I can not hold a pen since the loss of my nails …"
During Edison's time, people would host x-ray parties in their homes. The host of these gatherings would allow guests to x-ray different parts of their body. People would leave these events with their framed souvenirs. X-rays were even used to measure foot size. Devices like the fluoroscope were placed in shoe stores. Technicians, consumers, and observers could look in oculars while the foot was being x-rayed. Unnecessary exposure to radiation was not considered as a hazard during these times. In this era, most believed this was the most accurate way to measure the foot.
Radioactivity
When Becquerel heard about Roentgen's discovery, he wondered if his fluorescent minerals would give the same x-rays. Becquerel placed some of his rock crystals on top of a well-covered photographic plate and sat them in the sunlight. The sunlight made the crystals glow with a bright fluorescent light, but when Becquerel developed the film he was very disappointed. He found that only one of his minerals, a uranium salt, had fogged the photographic plate. He decided to try again, and this time, to leave them out in the sun for a longer period of time. Fortunately, the weather did not cooperate and Becquerel had to leave the crystals and film stored in a drawer for several cloudy days. Before continuing his experiments, Becquerel decided to check one of the photographic plates to make sure the chemicals were still good. To his amazement, he found that the plate had been exposed in spots where it had been near the uranium containing rocks and some of these rocks had not been exposed to sunlight at all. In later experiments, Becquerel confirmed that the radiation from the uranium had no connection with light or fluorescence, but the amount of radiation was directly proportional to the concentration of uranium in the rock. Becquerel had discovered radioactivity.

The Curies and Radium
One of Becquerel's assistants, a young Polish scientist named Maria Sklowdowska (to become Marie Curie after she married Pierre Curie), became interested in the phenomenon of radioactivity. With her husband, she decided to find out if chemicals other than uranium were radioactive. The Austrian government was happy to send the Curies a ton of pitchblende from the mining region of Joachimstahl because it was waste material that had to be disposed of anyway. The Curies wanted the pitchblende because it was the residue of uranium mining. From the ton of pitchblende, the Curies separated \(0.10 \: \text{g}\) of a previously unknown element, radium, in the form of the compound radium chloride. This radium was many times more radioactive than uranium.
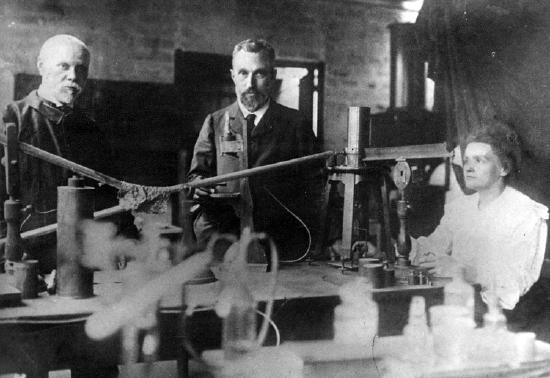
By 1902, the world was aware of a new phenomenon called radioactivity and of new elements that exhibited natural radioactivity. For this work, Becquerel and the Curies shared the 1903 Nobel Prize in Physics. These three researchers continued to work with hazardous radioactive materials. They experienced various ailments including burns and weakness by touching these substances. In 1906, Pierre Curie accidentally fell under a horse-drawn cart. He immediately died from his wounds and left Marie with two small children (Irene, 9 years old and Eve, 2 years old). After his death, Marie continued her research by identifying a new element, Polonium. In 1911, she was awarded the Nobel Prize in Chemistry for the discoveries of radium and polonium. As of today, Marie Curie is the only person ever to have received two Nobel Prizes in the sciences. To learn more about this famous female scientist, click on this link to watch a short video biography of her life.
Further experiments provided information about the characteristics of the penetrating emissions from radioactive substances. It was soon discovered that there were three common types of radioactive emissions. Some of the radiation could pass easily through aluminum foil while some of the radiation was stopped by the foil. Some of the radiation could even pass through foil up to a centimeter thick. The three basic types of radiation were named alpha, beta, and gamma radiation. The actual composition of the three types of radiation was still not known.
Eventually, scientists were able to demonstrate experimentally that the alpha particle, \(\alpha\), was a helium nucleus (a particle containing two protons and two neutrons), a beta particle, \(\beta\), was a high speed electron, and gamma rays, \(\gamma\), were a very high energy form of light (even higher energy than x-rays).
Unstable Nuclei May Disintegrate
A nucleus (with one exception, hydrogen-1) consists of some number of protons and neutrons pulled together in an extremely tiny volume. Since protons are positively charged and like charges repel, it is clear that protons cannot remain together in the nucleus unless there is a powerful force holding them there. The force which holds the nucleus together is generated by nuclear binding energy.
A nucleus with a large amount of binding energy per nucleon (proton or neutron) will be held together tightly and is referred to as stable. These nuclei do not break apart. When there is too little binding energy per nucleon, the nucleus will be less stable and may disintegrate (come apart). Such disintegrations are referred to as natural radioactivity. It is also possible for scientists to smash nuclear particles together and cause nuclear reactions between normally stable nuclei. These disintegrations are referred to as artificial radioactivity. None of the elements above #92 on the periodic table occur on earth naturally; they are all products of artificial radioactivity (man-made).
When nuclei come apart, they come apart violently accompanied by a tremendous release of energy in the form of heat, light, and radiation. This energy comes from some of the nuclear binding energy. In nuclear changes, the energy involved comes from the nuclear binding energy. However, in chemical reactions, the energy comes from electrons moving energy levels. A typical nuclear change (such as fission) may involve millions of times more energy per atom changing compared to a chemical change (such as burning)!
Need More Practice?
- Turn to Section 5.E of this OER and answer questions #5 and #7.
Contributors and Attributions
- Wikibooks


