4.11: Applications and Solubility of Covalent Compounds
- Page ID
- 96876
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Know the basic difference between the terms polar and nonpolar.
- Apply the like dissolves like solubility rule for covalent compounds
- Understand why molecular shapes are important to pharmaceutical chemists and biologists.
- Appreciate how medications/vitamins need to be soluble in the body.
- Pick a vitamin and memorize highlighted information
- Realize that molecules are three-dimensional.
- Know the applications of covalent compounds in this section
The Solubility of Covalent Compounds
Unlike ionic solubility, covalent compound solubility cannot be determined by a table. Instead, structures and three-dimensional shapes must be drawn. Once a correct geometry has been determined, the compound would be classified as being polar or nonpolar. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. Covalent solubility uses the like-dissolves-like rule. This means that substances with the same type of polarity will be soluble in one another. Moreover, compounds with differing polarities will be insoluble in one another.
Oil and water form a heterogeneous mixture due to their differing polarities; these substances are immiscible (not mixable) (Figure \(\PageIndex{1a}\)). In contrast, alcohol dissolves in water to form a homogeneous mixture (Figure \(\PageIndex{1b}\)).

In this class, we will not explore molecular geometries that are used to determine polarity. Instead, the polarity of a substance will be provided. It is important to remember that water is polar and oil/fat is nonpolar. If the polarity of a substance is given, you should be able to classify it as being water or oil/fat soluble.
In determining polarity, chemists look to the power of atom's' nucleus. The protons from an atom's nucleus are capable of attracting another atom's electrons. Within a covalent bond, valence electrons are pulled toward's an atom that has a more powerful nucleus. This pull is called electronegativity. If different atoms are connected in a bond, then one tends to be more electronegative than the other. Molecules that have an overall pull in one direction are labeled as being polar species. Look at the structure of water that is shown below. This structure of this molecule shows the bonding electrons being pulled towards oxygen. Therefore, oxygen has a more powerful nucleus than hydrogen. Water's bent molecular shape does not cancel out the individual dipole pulls. As a result, water has an overall pull and is classified as being polar.
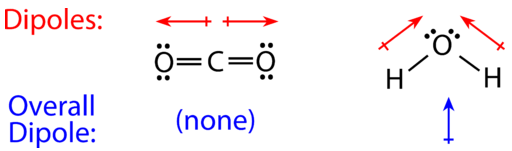
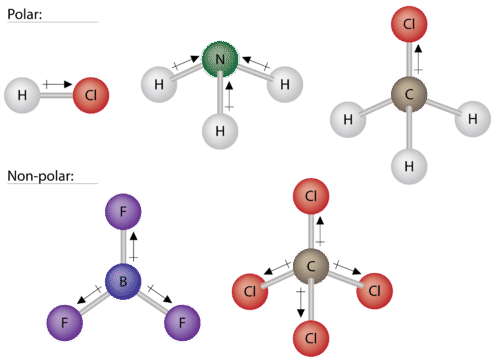
Carbon dioxide also has local dipoles (arrows) that pull in opposing directions. This molecule does not have an overall pull in one direction and is classified as being nonpolar. Some other molecules are shown in the figure below. The top three asymmetrical molecules are all polar. They all have dipoles (pulls) that do not cancel. All of these molecules would be soluble in water. The bottom three molecules are nonpolar. These are symmetrical molecules that have dipoles that cancel. Both of these molecules would be oil or fat-soluble.
Covalent solubility is important in the pharmaceutical industry. If a medication is not water soluble, then it will not dissolve in the bloodstream and react in the active site of the body in a timely and potent fashion. Watch the video below to obtain a basic understanding of how ibuprofen travels through the body to reduce pain and/or inflammation.
 The polarity of vitamins can affect how long they remain in the body. Vitamins B and C are both water soluble and remain in the body for a short period of time. Our bodies must intake these vitamins more frequently. On the other hand, vitamins A, D, E, and K are all fat or oil soluble. Nutrients that are fat soluble remain in the body longer. These vitamins accumulate easily and can be toxic if recommended doses are surpassed.
The polarity of vitamins can affect how long they remain in the body. Vitamins B and C are both water soluble and remain in the body for a short period of time. Our bodies must intake these vitamins more frequently. On the other hand, vitamins A, D, E, and K are all fat or oil soluble. Nutrients that are fat soluble remain in the body longer. These vitamins accumulate easily and can be toxic if recommended doses are surpassed.
Pick one vitamin from the table below and memorize the common/chemical names, one source, and one function. In addition, note the solubility (water or fat) of your selected vitamin.
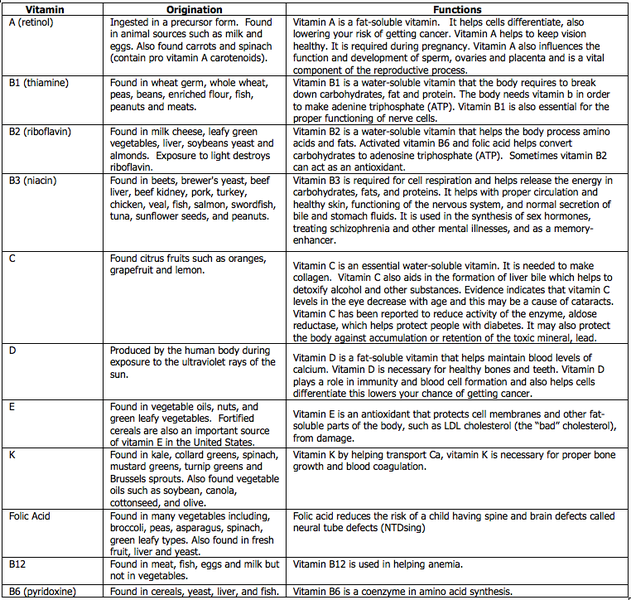
Figure \(\PageIndex{4}\): Vitamins with their sources and functions. Image taken from: upload.wikimedia.org/wikiped...mins_Table.png



Applications of Covalent Compounds
Carbon dioxide is the gas that all living animals and humans exhale. In addition, it is also produced in the combustion process of fossil fuels (natural gas, coal, and petroleum products) and the burning of materials (wood). This covalent molecule is one of many global warming gases. The Environmental Protection Agency (EPA) does not regulate carbon dioxide emissions for industrial manufacturing or energy-producing facilities.
![]()
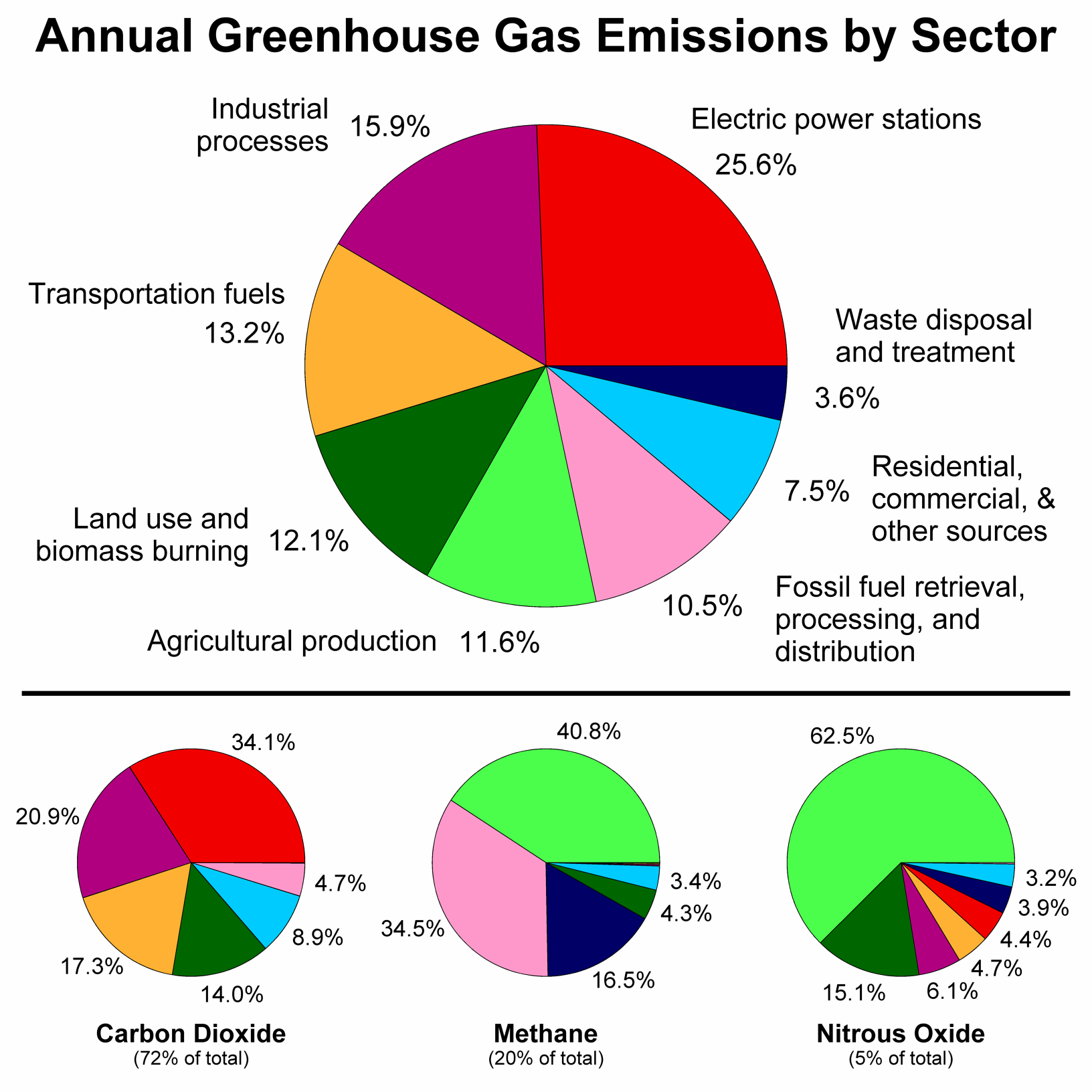
![]() Figure \(\PageIndex{4}\): Annual greenhouse gases by sector. Image taken from: https://images.app.goo.gl/gCZT9sNY4jG3VtkdA
Figure \(\PageIndex{4}\): Annual greenhouse gases by sector. Image taken from: https://images.app.goo.gl/gCZT9sNY4jG3VtkdA
Carbon monoxide results from the incomplete burning of wood and fossil fuels. During the combustion process, both carbon monoxide and carbon dioxide are produced. If burning occurs in an environment deficient in oxygen concentration, then more carbon monoxide is produced. In addition, burning in a colder temperature setting allows carbon monoxide to increase in an area. When burning any type of fuel, it is important to allow fumes to ventilate and not collect in a closed environment. For example, you should not sit in your garage with your car engine running. It is safer to crank your vehicle and immediately move it out of the confined space of your garage.
.jpg?revision=1) Figure \(\PageIndex{5}\): Symptoms of carbon monoxide poisoning. Image taken from:CO toxicity symptoms (en) - Carbon monoxide poisoning - Wikipedia. This file was derived from: Simptomi trovanja CO.jpg:
Figure \(\PageIndex{5}\): Symptoms of carbon monoxide poisoning. Image taken from:CO toxicity symptoms (en) - Carbon monoxide poisoning - Wikipedia. This file was derived from: Simptomi trovanja CO.jpg: If you have gas-powered energy or portable combustible fuel space heaters, it is important to have a carbon monoxide detector. These types of devices should be used in addition to a regular smoke detector. Carbon monoxide is an odorless, tasteless, and colorless gas. Exposure to this chemical can mimic a stomach virus. Unfortunately, carbon monoxide kills quickly with little warning. In terms of air quality, the EPA monitors and sets limits for industrial carbon monoxide production.
Dihydrogen monosulfide is a byproduct of the petroleum industry. In addition, paper mills, tanneries, sewage treatment factories, and coke ovens emit this gas into the atmosphere. People expel dihydrogen monosulfide when they pass gas. In nature, volcanoes continuously make this and other sulfur-based gases. This colorless and corrosive gas smells like rotten eggs.
SOx (sulfur dioxide and sulfur trioxide gases) result from the combustion of sulfur-based fuels (coal and diesel). Like H2S(g), volcanoes produce these two sulfur-based gases. Cleaner-based fossil fuels reduce the amount of sulfur based compounds before burning. SOx gases can combine with water to produce acid rain. If rain water has a pH equal to or below 5.6, the EPA classifies this precipitation as being acid rain (on a normal pH scale an acid pH would be equal to or below 6.9). Acid rain can destroy plant life, dissolve materials like marble, and lower the pH of aquatic environments. For more information regarding the harmful effects of acid rain, click here and explore the EPA's webpage.
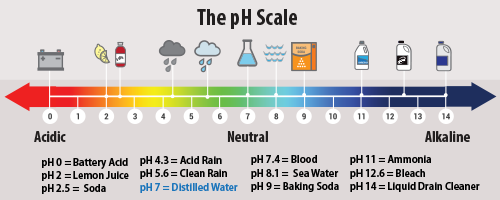
SOx gases can combine with particles to form a black-brown color that is called industrial smog. This type of pollution is commonly seen in more urban and industrialized areas. The burning of coal and diesel will reduce the air quality in a region where it is heavily used. For these reasons, the petroleum-based SOxx gas production for all industries in the United States.
All types of burning produce NOx (nitrogen monoxide and nitrogen dioxide) gases. The combustion of wood, gasoline, diesel, natural gas, coal, and other petroleum base products will produce NOxs. Lightning naturally emits these compounds as well. Environmental problems associated with NOxs are photochemical smog and acid rain. Areas with high concentrations of NOx typically have a large amount of mobile sources (cars, trains, and trucks) and industrial plants. For more information about NOx, click on this EPA link. Like SOx and carbon monoxide, the EPA regulates industrial emissions of NOx.
Isabella Quiros (Furman University)



