15.10.4: Cholesterol Metabolism
- Page ID
- 337691
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The cholesterol biosynthesis pathway is a long one and it requires significant amounts of reductive and ATP energy, which is why it is included here. Cholesterol has important roles in the body in membranes. It as also a precursor of steroid hormones and bile acids and its immediate metabolic precursor, 7-dehydrocholesterol, is a precursor of Vitamin D. The pathway leading to cholesterol is known as the isoprenoid pathway and branches of it lead to other molecules including other fat-soluble vitamins.
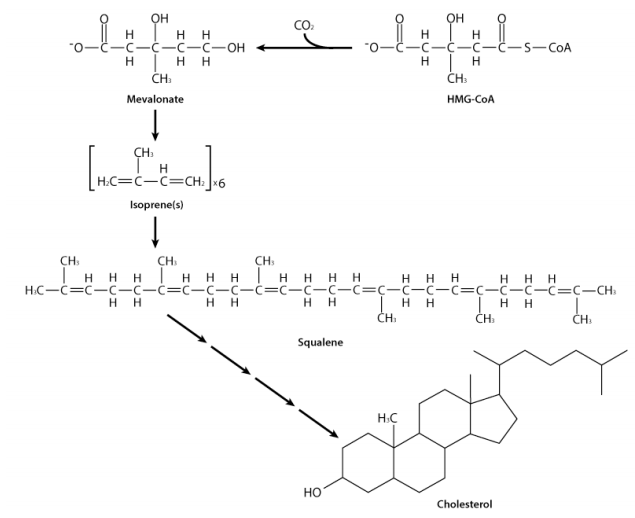 Figure 6.8.1: The pathway to cholesterol
Figure 6.8.1: The pathway to cholesterol
From HMG-CoA, the enzyme HMG-CoA reductase catalyzes the formation of mevalonate. The reaction requires NADPH and results in release of coenzyme A and appears to be one of the most important regulatory steps in the synthesis pathway. The enzyme is regulated both by feedback inhibition (cholesterol inhibits it) and by covalent modification (phosphorylation inhibits it). The enzyme’s synthesis is also regulated transcriptionally. When cholesterol levels fall, transcription of the gene increases.
Mevalonate gets phosphorylated twice and then decarboxylated to yield the five carbon intermediate known as isopentenyl-pyrophosphate (IPP). IPP is readily converted to dimethylallylpyrophosphate (DMAPP). These two five carbon compounds, also called isoprenes, are the building blocks for the synthesis of cholesterol and related compounds. This pathway is known as the isoprenoid pathway. It proceeds in the direction of cholesterol starting with the joining of IPP and DMAPP to form geranyl-pyrophosphate. Geranyl-pyrophosphate combines with another IPP to make farnesyl-pyrophosphate, a 15-carbon compound. Two farnesyl-pyrophosphates join to create the 30-carbon compound known as squalene. Squalene, in a complicated rearrangement involving reduction and molecular oxygen forms a cyclic intermediate known as lanosterol that resembles cholesterol. Conversion of lanosterol to cholesterol is a lengthy process involving 19 steps that occur in the endoplasmic reticulum.
Branching from cholesterol, one can form Vitamin D or the steroid hormones, which include the progestagens, androgens, estrogens, mineralocorticoids, and the glucocorticoids. The branch molecule for all of these is the cholesterol metabolite (and progestagen) known as pregnenalone. The progestagens are precursors of all of the other classes.
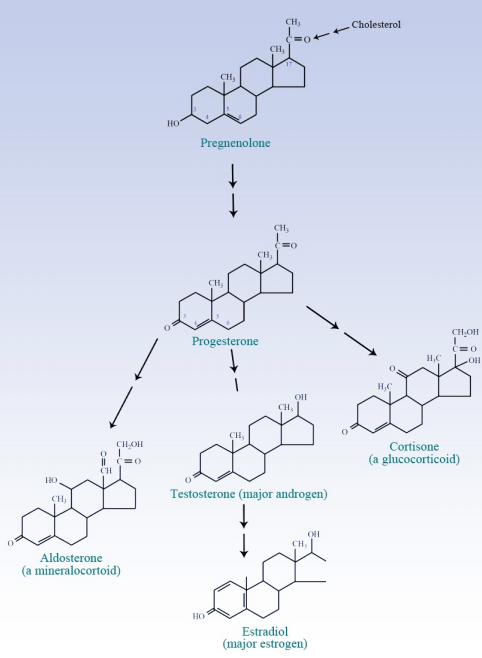 Figure 6.8.2: Steroid Hormone Synthesis
Figure 6.8.2: Steroid Hormone Synthesis
The estrogens are derived from the androgens in an interesting reaction that required formation of an aromatic ring. The enzyme catalyzing this reaction is known as an aromatase and it is of medical significance. The growth of some tumors is stimulated by estrogens, so aromatase inhibitors are prescribed to prevent the formation of estrogens and slow tumor growth. It is worth noting that synthesis of other fat soluble vitamins and chlorophyll also branches from the isoprenoid synthesis pathway at geranylpyrophosphate. Joining of two geranylgeranylpyrophosphates occurs in plants and bacteria and leads to synthesis of lycopene, which, in turn is a precursor of beta-carotene, the final precursor of Vitamin A. Vitamins E and K, as well as chlorophyll are all also synthesized from geranylgeranylpyrophosphate.
Bile Acid Metabolism
Another pathway from cholesterol leads to the polar bile acids, which are important for the solubilization of fat during digestion. Converting the very non-polar cholesterol to a bile acid involves oxidation of the terminal carbon on the side chain off the rings. Other alterations to increase the polarity of these compounds include hydroxylation of the rings and linkage to other polar compounds.
Common bile acids include cholic acid, chenodeoxycholic acid, glycocholic acid, taurocholic acid, and deoxycholic acid. Another important fact about bile acids is that their synthesis reduces the amount of cholesterol available and promotes uptake of LDLs by the liver. Normally bile acids are recycled efficiently resulting in limited reduction of cholesterol levels. However, inhibitors of the recycling promote reduction of cholesterol levels.
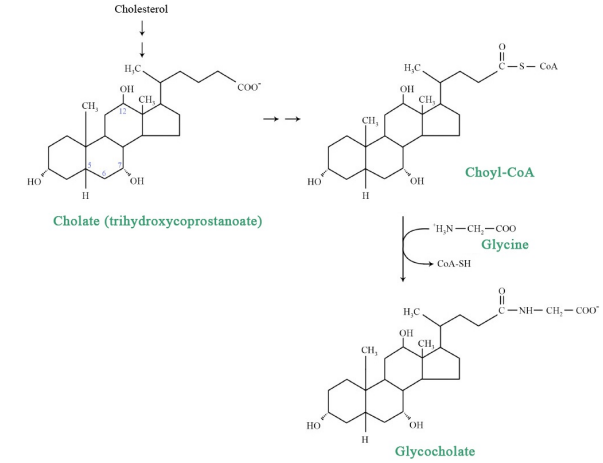 Figure 6.8.3: Bile Salts
Figure 6.8.3: Bile Salts


