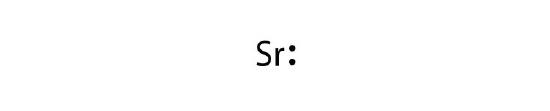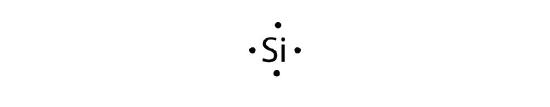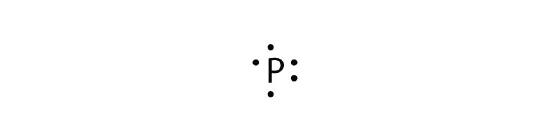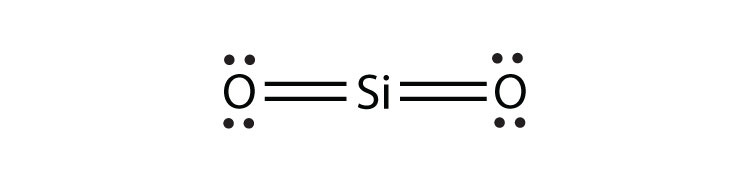5.E: Chemical Bonds (Exercises)
- Page ID
- 390455
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)5.2: Lewis Electron Dot Diagrams
- Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
- Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol?
- What column of the periodic table has Lewis electron dot diagrams with two electrons?
- What column of the periodic table has Lewis electron dot diagrams that have six electrons in them?
- Draw the Lewis electron dot diagram for each element.
- strontium
- silicon
- Draw the Lewis electron dot diagram for each element.
- krypton
- sulfur
- Draw the Lewis electron dot diagram for each element.
- titanium
- phosphorus
- Draw the Lewis electron dot diagram for each element.
- bromine
- gallium
- Draw the Lewis electron dot diagram for each ion.
- Mg2+
- S2−
- Draw the Lewis electron dot diagram for each ion.
- In+
- Br−
- Draw the Lewis electron dot diagram for each ion.
- Fe2+
- N3−
- Draw the Lewis electron dot diagram for each ion.
- H+
- H−
Answers
- The first two electrons in a valence shell are s electrons, which are paired.
- the second column of the periodic table
-
-
-
- Mg2+
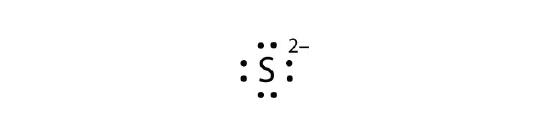
-
- Fe2+
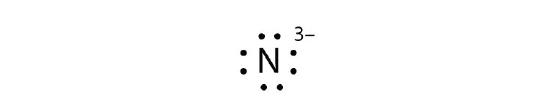
5.3: Electron Transfer - Ionic Bonds
- Comment on the possible formation of the K2+ ion. Why is its formation unlikely?
-
Comment on the possible formation of the Cl2− ion. Why is its formation unlikely?
-
How many electrons does a Ba atom have to lose to have a complete octet in its valence shell?
-
How many electrons does a Pb atom have to lose to have a complete octet in its valence shell?
-
How many electrons does an Se atom have to gain to have a complete octet in its valence shell?
-
How many electrons does an N atom have to gain to have a complete octet in its valence shell?
-
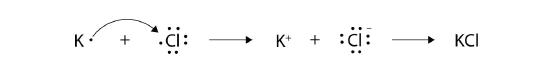
With arrows, illustrate the transfer of electrons to form potassium chloride from K atoms and Cl atoms.
-
With arrows, illustrate the transfer of electrons to form magnesium sulfide from Mg atoms and S atoms.
-
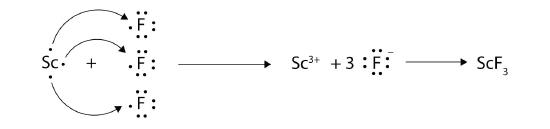
With arrows, illustrate the transfer of electrons to form scandium fluoride from Sc atoms and F atoms.
-
With arrows, illustrate the transfer of electrons to form rubidium phosphide from Rb atoms and P atoms.
-
Which ionic compound has the higher lattice energy—KI or MgO? Why?
-
Which ionic compound has the higher lattice energy—KI or LiF? Why?
- Which ionic compound has the higher lattice energy—BaS or MgO? Why?
Answers
- The K2+ ion is unlikely to form because the K+ ion already satisfies the octet rule and is rather stable.
- two
- two
- MgO because the ions have a higher magnitude charge
- MgO because the ions are smaller
5.4: Covalent Bonds
- How many electrons will be in the valence shell of H atoms when it makes a covalent bond?
- How many electrons will be in the valence shell of non-H atoms when they make covalent bonds?
- What is the Lewis electron dot diagram of I2? Circle the electrons around each atom to verify that each valence shell is filled.
- What is the Lewis electron dot diagram of H2S? Circle the electrons around each atom to verify that each valence shell is filled.
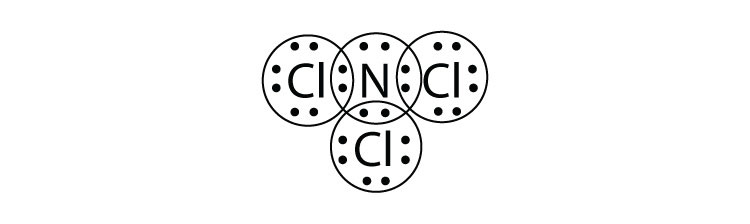
- What is the Lewis electron dot diagram of NCl3? Circle the electrons around each atom to verify that each valence shell is filled.
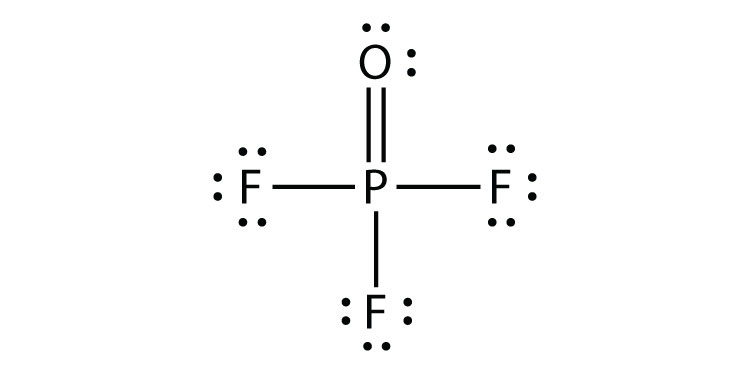
- What is the Lewis electron dot diagram of SiF4? Circle the electrons around each atom to verify that each valence shell is filled.
- Draw the Lewis electron dot diagram for each substance.
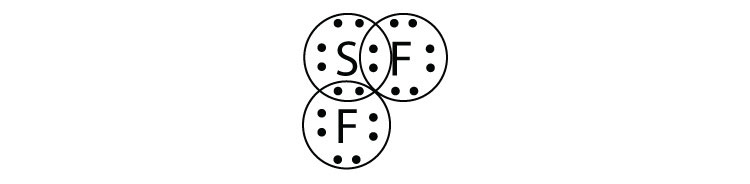
- SF2
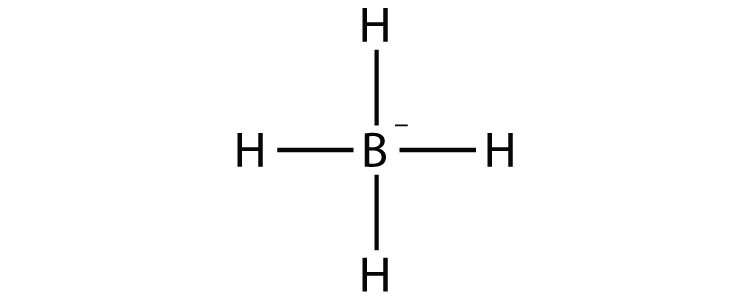
- BH4−
- Draw the Lewis electron dot diagram for each substance.
- PI3
- OH−
- Draw the Lewis electron dot diagram for each substance.
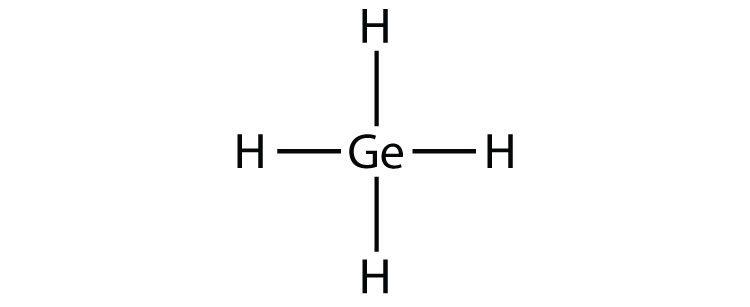
- GeH4
- ClF
- Draw the Lewis electron dot diagram for each substance.
- AsF3
- NH4+
- Draw the Lewis electron dot diagram for each substance. Double or triple bonds may be needed.
- SiO2
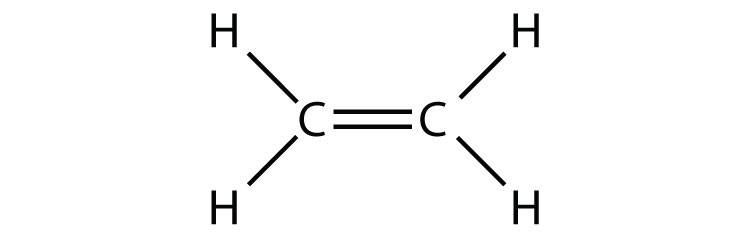
- C2H4 (assume two central atoms)
- Draw the Lewis electron dot diagram for each substance. Double or triple bonds may be needed.
- CN−
- C2Cl2 (assume two central atoms)
- Draw the Lewis electron dot diagram for each substance. Double or triple bonds may be needed.
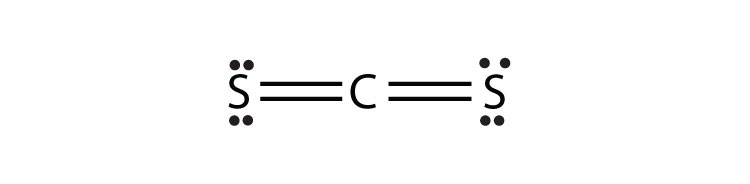
- CS2
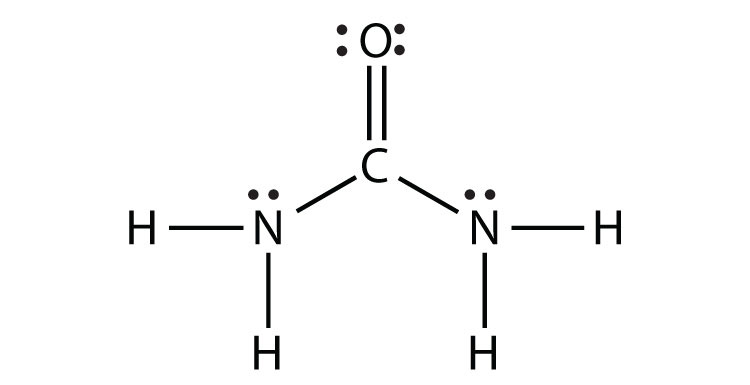
- NH2CONH2 (assume that the N and C atoms are the central atoms)
- Draw the Lewis electron dot diagram for each substance. Double or triple bonds may be needed.
- POCl
- HCOOH (assume that the C atom and one O atom are the central atoms)
Answers
- two

-
-
-
-
5.5: Other Aspects of Covalent Bonds
- Give an example of a nonpolar covalent bond. How do you know it is nonpolar?
- Give an example of a polar covalent bond. How do you know it is polar?
- How do you know which side of a polar bond has the partial negative charge? Identify the negatively charged side of each polar bond.
- H–Cl
- H–S
- How do you know which side of a polar bond has the partial positive charge? Identify the positively charged side of each polar bond.
- H–Cl
- N-F
- Label the bond between the given atoms as nonpolar covalent, polar covalent, or likely ionic.
- F and F
- C and F
- K and F
- Label the bond between the given atoms as nonpolar covalent, slightly polar covalent, definitely polar covalent, or ionic.
- S and Cl
- P and O
- Cs and O
- Which covalent bond is stronger—a C–C bond or a C–H bond?
- Which covalent bond is stronger—an O–O double bond or an N–N double bond?
- Estimate the enthalpy change for this reaction: N2 + 3H2 → 2NH3 .Start by drawing the Lewis electron dot diagrams for each substance.
- Estimate the enthalpy change for this reaction. Start by drawing the Lewis electron dot diagrams for each substance: HN=NH + 2H2 → 2NH3
- Estimate the enthalpy change for this reaction. Start by drawing the Lewis electron dot diagrams for each substance: CH4 + 2O2 → CO2 + 2H2O
- Estimate the enthalpy change for this reaction. Start by drawing the Lewis electron dot diagrams for each substance: 4NH3 + 3O2 → 2N2 + 6H2O
Answers
- H–H; it is nonpolar because the two atoms have the same electronegativities (answers will vary).
-
- Cl side
- S side
-
- nonpolar covalent
- polar covalent
- ionic
- C–H bond
- −80 kJ
- −798 kJ
5.6: Violations of the Octet Rule
- Why can an odd-electron molecule not satisfy the octet rule?
- Why can an atom in the second row of the periodic table not form expanded valence shell molecules?
- Draw an acceptable Lewis electron dot diagram for these molecules that violate the octet rule.
- NO2
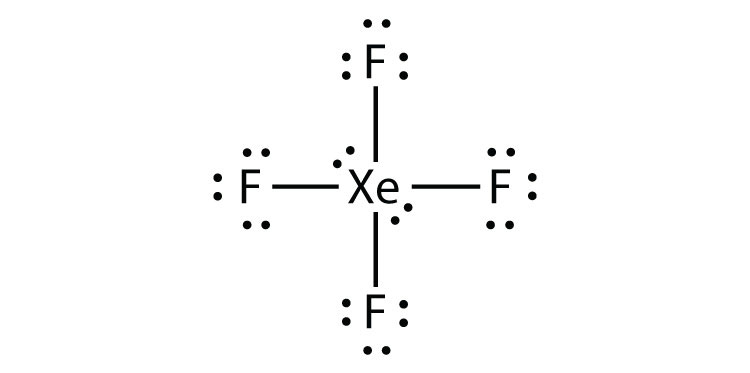
- XeF4
- Draw an acceptable Lewis electron dot diagram for these molecules that violate the octet rule.
- BCl3
- ClO2
- Draw an acceptable Lewis electron dot diagram for these molecules that violate the octet rule.
- POF3
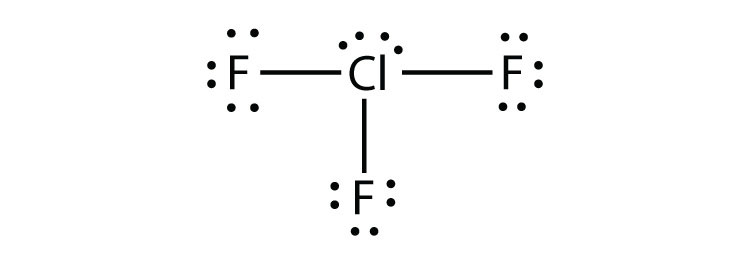
- ClF3
- Draw an acceptable Lewis electron dot diagram for these molecules that violate the octet rule.
- SF4
- BeH2
Answers
- There is no way all electrons can be paired if there are an odd number of them.
-
-
5.7: Molecular Shapes
- What is the basic premise behind VSEPR?
- What is the difference between the electron group geometry and the molecular geometry?
- Identify the molecular geometry of each molecule.
- H2S
- POCl3
- Identify the electron group geometry and the molecular geometry of each molecule.
- CS2
- H2S
- Identify the molecular geometry of each molecule.
- HCN
- CCl4
- Identify the electron group geometry and the molecular geometry of each molecule.
- BI3
- PH3
- What is the molecular geometry of each species?
- CN−
- PO43−
- What is the geometry of each species?
- PO33−
- NO3−
- What is the geometry of each species?
- COF2
- C2Cl2 (both C atoms are central atoms and are bonded to each other)
- What is the geometry of each species?
- CO32−
- N2H4 (both N atoms are central atoms and are bonded to each other)
Answers
- Electron pairs repel each other.
-
- molecular geometry: bent
- molecular geometry: tetrahedral
-
- molecular geometry: linear
- molecular geometry: tetrahedral
-
- linear
- tetrahedral
-
- trigonal planar
- linear and linear about each central atom
5.8 Additional Exercises
- Explain why iron and copper have the same Lewis electron dot diagram when they have different numbers of electrons.
-
Name two ions with the same Lewis electron dot diagram as the Cl− ion.
-
Based on the known trends, what ionic compound from the first column of the periodic table and the next-to-last column of the periodic table should have the highest lattice energy?
-
Based on the known trends, what ionic compound from the first column of the periodic table and the next-to-last column of the periodic table should have the lowest lattice energy?
-
P2 is not a stable form of phosphorus, but if it were, what would be its likely Lewis electron dot diagram?
-
Se2 is not a stable form of selenium, but if it were, what would be its likely Lewis electron dot diagram?
-
What are the Lewis electron dot diagrams of SO2, SO3, and SO42−?
-
What are the Lewis electron dot diagrams of PO33− and PO43−?
-
Which bond do you expect to be more polar—an O–H bond or an N–H bond?
-
Which bond do you expect to be more polar—an O–F bond or an S–O bond?
-
Use bond energies to estimate the energy change of this reaction.
C3H8 + 5O2 → 3CO2 + 4H2O -
Use bond energies to estimate the energy change of this reaction.
N2H4 + O2 → N2 + 2H2O -
Ethylene (C2H4) has two central atoms. Determine the geometry around each central atom and the shape of the overall molecule.
-
Hydrogen peroxide (H2O2) has two central atoms. Determine the geometry around each central atom and the shape of the overall molecule.
Answers
- Iron has d electrons that typically are not shown on Lewis electron dot diagrams.
-
-
LiF
-
-
It would be like N2:
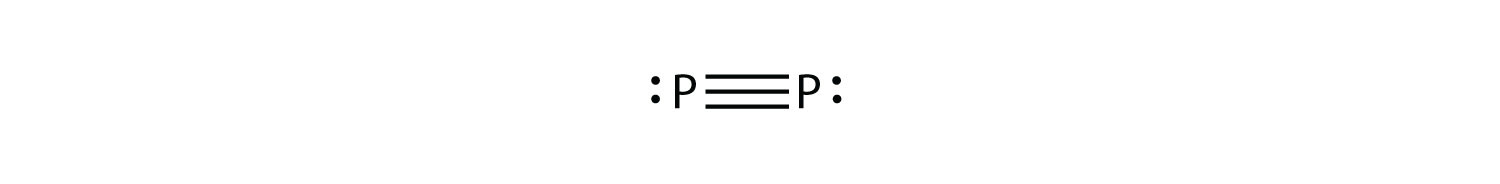
-
-
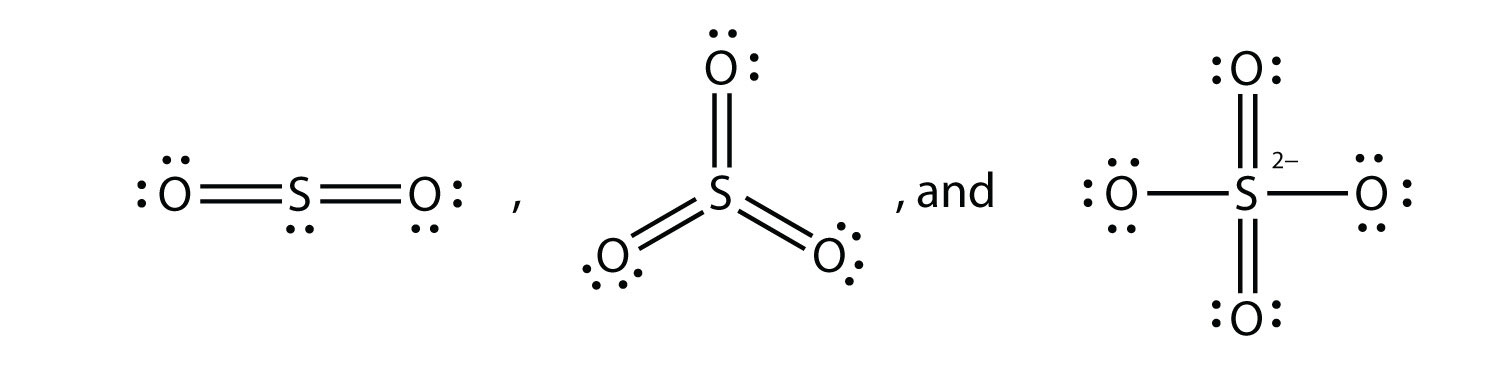
-
-
an O–H bond
-
-
−2,000 kJ
-
-
trigonal planar about both central C atoms